Академический Документы
Профессиональный Документы
Культура Документы
Biomolecules See The Light: Scaffold Proteins Activating Enzymes Phosphatases Substrates
Загружено:
JonathanОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Biomolecules See The Light: Scaffold Proteins Activating Enzymes Phosphatases Substrates
Загружено:
JonathanАвторское право:
Доступные форматы
S C I E N C E ’ S C O M PA S S
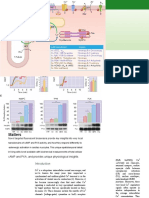
65 Docking groove crystal structures (see the first figure). region of MAPKK, blocks MAPK function
64 CD site Thus, the ED site may aid the docking during anthrax infection (9, 10). The proteol-
63 ED site of some interacting proteins, although ysis separates the NH2-terminal D domain of
MAPK Scaffold
62 Active site proteins the mutational analysis used to identi- MAPKK from the catalytic kinase domain
61 fy the ED site may have indirectly af- that phosphorylates and activates MAPK (9).
60 Activating fected the docking groove identified Loss of the D domain prevents recognition
59 enzymes by crystallography. of the cognate MAPK by the cleaved
58 Chang and colleagues unexpected- MAPKK. This observation could lead to
57 Phosphatases ly observed that the binding of pro- the use of small molecules (that is, drugs)
56 teins to D domains induces conforma- to disrupt MAPK interactions and to block
Substrates
55 tional changes in p38 MAPK. The selectively individual MAPK pathways.
54 largest change occurs in the loop be- The structural insights of Chang and
53 tween αd and αe, which narrows the colleagues will provide a foundation for
52 MAP kinase interactions with scaffold molecules, binding groove between these helices future studies of the molecular basis of
MAPKKs, phosphatases, and substrates.
51 and the β7-β8 reverse turn. The bind- MAPK signaling specificity. These studies
50 ing and conformational changes that will further our understanding of MAPK
49 natively, the CD site interaction may occur MEF2A and MKK3b induce differ and may signaling networks in health and disease.
48 only with activated MAPK or full-length contribute to p38 regulation. For example,
47 proteins; the structures reported by Chang alterations in the p38 activation loop caused References
46 and colleagues include nonactivated p38 by docking to MKK3b could aid p38 phos- 1. A. D. Sharrocks et al., Trends Biochem. Sci. 25, 448
45 MAPK and isolated D domains. A second phorylation, and the conformational (2000).
2. H. Enslen, R. J. Davis, Biol. Cell 93, 5 (2001).
44 MAPK site implicated in the interaction of changes caused by docking MEF2A may 3. C. I. Chang et al., Mol. Cell 9, 1241 (2002).
43 MAPKs with MAPK-activated protein ki- activate p38. 4. R. J. Davis, Cell 103, 239 (2000).
42 nases (MAPKAPKs), the ED site (7), also Disease research may benefit from un- 5. S. H. Yang et al., Mol. Cell. Biol. 19, 4028 (1999).
41 did not participate in the interaction of p38 derstanding the mechanisms that regulate 6. S. H. Yang et al., EMBO J. 17, 1740 (1998).
7. T. Tanoue et al., EMBO J. 20, 466 (2001).
40 MAPK with MEF2A or MKK3b in the MAPK signaling specificity. For example, 8. T. Tanoue et al., Nature Cell Biol. 2, 110 (2000).
39 crystal structures (3). The ED site, however, lethal factor (LF), a protease that binds to 9. N. S. Duesbery et al., Science 280, 734 (1998).
38 is located close to the docking groove in the and proteolytically cleaves the NH2-terminal 10. A. D. Pannifer et al., Nature 414, 229 (2001).
37
36 P E R S P E C T I V E S : M O L E C U L A R DY N A M I C S
35 calculated to have 164 minima connected
34 by 714 transition states, with 65 minima ly-
33
32
Biomolecules See the Light ing within 40 kJ/mol of the global mini-
mum (6). Different minima correspond to
31 David W. Pratt geometries with extended or partially fold-
30 ed peptide chains. Comparison of the rela-
29 olecular-level understanding of lated a biomolecule in the gas phase, and tive abundances of the different conforma-
28
27
26
M the complex dynamics of biologi-
cal processes such as protein fold-
ing will greatly advance the treatment of
identified and determined the relative popu-
lations of its energetically accessible “con-
formational substates” under collision-free
tions before and after IR excitation pro-
vides information about how a given struc-
ture evolves along a particular pathway.
25 human disease. Rapid progress toward this conditions. They then manipulated these The experiment of Dian et al. (5) is illus-
24 goal has been made in the past few years, populations with tunable laser light, using trated schematically in the figure. NATMA
23 owing to advances in experimental and collisions to relax the molecules back into was heated to 150°C, entrained in helium,
22 theoretical techniques (1–3). For example, their lowest energy conformations after they and passed through a ~1-mm orifice into a
21 multidimensional nuclear magnetic reso- had visited less favorable regions of the en- vacuum chamber, creating a supersonic ex-
20 nance techniques have helped to determine ergy landscape. Surprisingly, they found pansion that cools each molecule into one of
19 the solution structures of large proteins that the resulting popula-
18 and probe their dynamical behavior (4). tion distributions depend
17 But existing approaches cannot fully upon the precise nature IR
16 elucidate the heterogeneity of biological of the excitation, demon-
15 systems, their intricate energy landscapes, strating for the first time UV
14 and the possible role of solvent in the dy- that there are distin- II
13 namics. On page 2369 of this issue, Dian guishable pathways for
12 et al. report the development of a new ap- conformational change.
11 proach that overcomes some of these limi- The authors studied A I
10 tations (5). The method provides surpris- a methyl-capped dipep- B
9 ingly detailed insights into the dynamics tide called NATMA (N- C III
CREDIT: PRESTON MORRIGHAN/SCIENCE
8 of a small biomolecule. acetyl-tryptophan
7 Using a supersonic jet, infrared (IR) and methylamide). The 36- Exploring the energy landscape with light. NATMA molecules expanded
6 ultraviolet (UV) lasers, and ab initio quan- atom molecule is small in a supersonic jet of helium are observed by UV spectroscopy (III) to exist
5 tum chemical calculations, the authors iso- by biomolecule stan- in three different conformational substates (A, B, and C), corresponding to
4 dards but nonetheless geometries with extended or partially folded side chains. Irradiating the
3 The author is at the Department of Chemistry, Uni- complex in its dynam- ensemble with IR light (I) and relaxing it by collisions (II) changes the pop-
2 versity of Pittsburgh, Pittsburgh, PA 15260, USA. E- ics. The energy land- ulation ratio in a conformation-specific way, demonstrating that unique
1 mail: pratt@pitt.edu scape of the molecule is pathways govern the folding dynamics of this dipeptide.
www.sciencemag.org SCIENCE VOL 296 28 JUNE 2002 2347
S C I E N C E ’ S C O M PA S S
65 its lowest energy structures (A, B, or C). The this is clearly not the case.” Instead, the IR-in- of water and other small molecules in influ-
64 resulting ensemble was then probed with duced dynamics is nonstatistical, an unprece- encing conformational choice can be probed
63 two lasers: an IR laser irradiating the mix- dented result in such a large molecule (10). by forming solvent-solute complexes in the
62 ture during the expansion (~1 mm from the Under the conditions of the experiment, supersonic jet (14). It may also be possible
61 nozzle), and a UV laser further downstream collisions must play an important role in the to determine the extent to which different
60 (~4 mm from the nozzle). The UV absorp- dynamics. Selective collisional cooling of pathways are connected and to probe the vi-
59 tion spectrum of the sample gives informa- molecules with flexible side-chain confor- brational coordinates along which these
58 tion about the relative populations of A, B, mations has been observed by several connections are made. Such experiments
57 and C in the mixture. Each structure exhibits groups [see, for example (11)]. In their ele- would provide stringent tests of the “new
56 a unique spectrum (7). When the IR laser gant “chemical timing” experiments, Par- view” that multiple routes dominate the dy-
55 was turned on and tuned to a specific NH menter and co-workers (12) arrested IVR in namics of protein folding and other complex
54 stretching vibration of a particular confor- an electronically excited state of p-difluo- biological processes (15, 16). The future of
53 mation (A, B, or C), the relative intensities robenzene by using an O2 buffer gas to relax biomolecules in the gas phase is bright.
52 of the three different bands in the UV spec- the molecules back to the ground state on
51 trum changed, providing evidence for an IR- short time scales. The first collisions after References and Notes
50 induced isomerization reaction. IR excitation of NATMA likely occur in less 1. J. R. Winkler, H. B. Gray, Eds., special issue on Protein
Folding, Acc. Chem. Res. 31 (1998).
49 It is not surprising that the relative pop- than a nanosecond. They may thus be simi- 2. C. M. Dobson, A. Sali, M. Karplus, Angew. Chem. Int.
48 ulations of A, B, and C change when the larly responsible for the observed nonstatis- Ed. 37, 868 (1998).
47 3. M. R. Betancourt, D. Thirumalai, J. Phys. Chem. B 106,
ensemble is irradiated by IR light. IR-in- tical behavior. More sophisticated ab initio 599 (2002).
46 duced isomerization of a polyatomic calculations (13) will be required to identify 4. R. Riek et al., Nature 382, 180 (1996).
45 molecule (nitrous acid, HONO, in a low- the regions of the energy landscape to which 5. B. C. Dian, A. Longarte, T. S. Zwier, Science 296, 2369
(2002); published online 23 May 2002 (10.1126/
44 temperature matrix) was first observed access is blocked in such large molecules. science.1071563).
43 more than 40 years ago (8). Real-time stud- The results of Dian et al. (5) clearly show 6. D. Evans, D. J. Wales, personal communication.
42 ies of protein folding often use IR pulses to that there are distinguishable pathways on the 7. T. S. Zwier, J. Phys. Chem. A 105, 8827 (2001).
8. J. D. Baldeschwieler, G. C. Pimentel, J. Chem. Phys. 33,
41 initiate conformational change, either di- energy landscape of NATMA, at least on 1008 (1960).
40 rectly or indirectly (9), thereby mimicking some time scales. These pathways play a crit- 9. I. K. Lednev, A. S. Karnoup, M. C. Sparrow, S. A. Asher, J.
39 the well-known behavior of proteins to de- ical role in dictating the redistribution of pop- Am. Chem. Soc. 123, 2388 (2001).
10. Nonstatistical behavior is frequently predicted (and
38 nature at elevated temperature. ulations under the conditions of the experi- observed) in the chemical reaction dynamics of small
37 What is surprising about the results of ment. We do not know the extent to which molecules. See (17) for a recent example.
36 11. P. D. Godfrey, R. D. Brown, F. M. Rogers, J. Mol. Struct.
Dian et al. (5) is that they are conformation this will be true in even larger molecules. But 376, 65 (1996).
35 specific. The substates (A, B, or C) popu- if such pathways exist and can be accessed, 12. R. A. Coveleskie, D. A. Dolson, C. S. Parmenter, J. Phys.
34 lated after IR excitation are the same as in then we can look forward to obtaining ever Chem. 89, 645 (1985).
13. G. M. Florio, R. A. Christie, K. D. Jordan, T. S. Zwier, J.
33 the absence of IR excitation. But the popu- more detailed information about biological Am. Chem. Soc., in press.
32 lation ratios A:B:C before and after IR irra- processes at the molecular level. 14. E. G. Robertson, J. P. Simons, Phys. Chem. Chem. Phys.
31 diation are different. Further, the experi- IR lasers provide an entry point to previ- 3, 1 (2001).
15. R. L. Baldwin, J. Biomol. NMR 5, 103 (1995).
30 ments show that the population ratios de- ously unexplored regions of the energy land- 16. K. A. Dill, H. S. Chan, Nature Struct. Biol. 4, 11 (1997).
29 pend uniquely on which conformation is scape. Solvent-induced changes and the role 17. L. Sun, K. Song, W. L. Hase, Science 296, 875 (2002).
28 excited, and on which NH stretching vibra-
27 tion is excited within a given conformation. P E R S P E C T I V E S : E VO L U T I O N
26 According to conventional wisdom, this
25
24
kind of mode specificity should not exist in
such a large molecule, at least not on the Chaperones
23 few-microsecond time scale of the experi-
22
21
ment. NATMA has 102 normal modes of
vibration. Some of these have relatively
as Buffering Agents?
20 high frequencies, such as the NH stretching Thomas Mitchell-Olds and Charles A. Knight
19 modes at ~3400 cm–1 (the energy of IR ex-
18 citation), but most have much lower fre- oes evolution draw on existing ge- phenotypic changes. Recently, heat shock
17
16
15
quencies of a few hundred cm–1 or less.
Hence, there is a high density of states at
the energy of excitation. It is further expect-
D netic variation in animals and plants
or must it wait around for new mu-
tations to arise? Sixty years ago, Wadding-
proteins (HSPs), a type of molecular chap-
erone, have been implicated in the genetic
buffering of the fruit fly Drosophila. Now,
14 ed that some couplings must exist between ton argued that cryptic genetic variation is Queitsch et al. (2) report in a recent issue
13 these modes. The energy deposited in the present for many traits, but that expression of Nature that the chaperone HSP90 pro-
12 NH stretching mode of a particular struc- of these variants under normal environ- vides genetic buffering in Arabidopsis and
11 ture should therefore be redistributed rapid- mental conditions is prevented by a process may contribute to the evolutionary adapta-
10 ly and irreversibly among all or most of the of “genetic buffering” (1). As Waddington tion of this plant.
9 remaining modes, a process known as in- demonstrated, stressful environmental con- HSPs are induced by high-temperature
8 tramolecular vibrational relaxation (IVR). ditions compromise the genetic buffering stress in organisms as diverse as bacteria, fun-
7 As stated by Dian et al. (5), “given that the system, leading to the breakdown of nor- gi, plants, and animals. These molecular chap-
6 three conformers (A, B, and C) have a similar mal development and enabling the expres- erones prevent irreversible aggregation of de-
5 energy (within ~2 kJ/mol) and receive nearly sion of cryptic genetic variation as visible natured proteins after heat or other protein-de-
4 identical amounts of energy in the vibrational naturing stresses. They also bind to a range of
3 excitation, one might have imagined that all The authors are at the Max Planck Institute for
client proteins that are crucial for regulating
2 transitions out of the three conformers would Chemical Ecology, Winzerlaer Strasse 10, Jena 07745, growth and development. The evolutionary
1 distribute their excited population similarly . . . Germany. E-mail: tmo@ice.mpg.de conservation of the heat shock response, and
2348 28 JUNE 2002 VOL 296 SCIENCE www.sciencemag.org
Вам также может понравиться
- Applications of Petroleum Geochemistry To Exploration and Reservoir Management PDFДокумент32 страницыApplications of Petroleum Geochemistry To Exploration and Reservoir Management PDFArmando Pinto FloresОценок пока нет
- The Good-Enough Sex Model For Couple Sexual SatisfactionДокумент13 страницThe Good-Enough Sex Model For Couple Sexual SatisfactionwernikОценок пока нет
- Ash Sap 2016Документ672 страницыAsh Sap 2016honeyworksОценок пока нет
- Bioenergetics DLLДокумент2 страницыBioenergetics DLLAlvin PaboresОценок пока нет
- Molecular Mechanisms in the Control of Gene ExpressionОт EverandMolecular Mechanisms in the Control of Gene ExpressionDonald P. NierlichОценок пока нет
- Gianna Pomata (Editor), Nancy G. Siraisi (Editor) - Historia - Empiricism and Erudition in Early Modern Europe (Transformations - Studies in The History of Science and Technology) (2006)Документ493 страницыGianna Pomata (Editor), Nancy G. Siraisi (Editor) - Historia - Empiricism and Erudition in Early Modern Europe (Transformations - Studies in The History of Science and Technology) (2006)Marcelo Rizzo100% (1)
- SOP - Chill Water Chemistry Management Rev AДокумент12 страницSOP - Chill Water Chemistry Management Rev Abenjiy80Оценок пока нет
- 2002-Signaling Pathways For PC12 Cell Differentiation - Making The Right ConnectionsДокумент3 страницы2002-Signaling Pathways For PC12 Cell Differentiation - Making The Right ConnectionsWim ZhuОценок пока нет
- Nri 2035Документ11 страницNri 2035ayumОценок пока нет
- Signaling Switches and Bistability Arising From Multisite Phosphorylation in Protein Kinase CascadesДокумент7 страницSignaling Switches and Bistability Arising From Multisite Phosphorylation in Protein Kinase Cascades1418767078Оценок пока нет
- The Polycomb Group Protein Yaf2 Regulates The Pluripotency of Embryonic Stem Cells in A Phosphorylation-Dependent MannerДокумент13 страницThe Polycomb Group Protein Yaf2 Regulates The Pluripotency of Embryonic Stem Cells in A Phosphorylation-Dependent MannerSHUMETОценок пока нет
- A Family of Lambda Phage cDNA Cloning VectorsДокумент10 страницA Family of Lambda Phage cDNA Cloning VectorsaaasidОценок пока нет
- In Silico Identification, Structure Prediction and Phylogenetic Analysis of The 2-O-Ribose Methyltransferase DomainДокумент8 страницIn Silico Identification, Structure Prediction and Phylogenetic Analysis of The 2-O-Ribose Methyltransferase DomainsserggiosОценок пока нет
- 1991 - Association of Myn, The Murine Homolog of Max, With C-Myc Stimulates Methylation-Sensitive DNA Binding and Ras CotransformationДокумент13 страниц1991 - Association of Myn, The Murine Homolog of Max, With C-Myc Stimulates Methylation-Sensitive DNA Binding and Ras CotransformationRaymond LaBoyОценок пока нет
- 1 s2.0 S0006349513015762 Main PDFДокумент1 страница1 s2.0 S0006349513015762 Main PDFDiego TulcanОценок пока нет
- Karouzaki Et Al. 2019Документ11 страницKarouzaki Et Al. 2019Christina MountakiОценок пока нет
- Biochimica Et Biophysica Acta: Alexander Plotnikov, Eldar Zehorai, Shiri Procaccia, Rony SegerДокумент15 страницBiochimica Et Biophysica Acta: Alexander Plotnikov, Eldar Zehorai, Shiri Procaccia, Rony Segerlinda ratna watiОценок пока нет
- Problema Cascada MAPK PDFДокумент1 страницаProblema Cascada MAPK PDFAriadna Vanesa MacíasОценок пока нет
- Crast A 2012Документ8 страницCrast A 2012Alim Wulandari KarimaОценок пока нет
- Snare Proteins and Membrane FusionДокумент3 страницыSnare Proteins and Membrane FusionNuman MajeedОценок пока нет
- Karow 2018Документ17 страницKarow 2018Laura Duarte RojasОценок пока нет
- Swi Ech 2014Документ9 страницSwi Ech 2014Emanuele GustaniОценок пока нет
- MoriguchiДокумент8 страницMoriguchiMARIA VICTORIA VELARDE ALIAGAОценок пока нет
- 2008 Palfreyman SNAREs PDFДокумент25 страниц2008 Palfreyman SNAREs PDFMădă IorgaОценок пока нет
- J. Biol. Chem.-1996-Kagoshima-33074-82Документ9 страницJ. Biol. Chem.-1996-Kagoshima-33074-82Vinod YadavОценок пока нет
- MAP Kinase Signalling Pathways in CancerДокумент12 страницMAP Kinase Signalling Pathways in Cancerpanna1Оценок пока нет
- Protein Acid NucleicДокумент2 страницыProtein Acid NucleicFlorin CazanОценок пока нет
- 2003-The Direct Activation of MIK, A Germinal Center Kinase (GCK) - LikeДокумент7 страниц2003-The Direct Activation of MIK, A Germinal Center Kinase (GCK) - LikeChao JiangОценок пока нет
- Forsberg Et Al 1994 Use of Transcriptional Fusions To Monitor Gene Expression A Cautionary TaleДокумент5 страницForsberg Et Al 1994 Use of Transcriptional Fusions To Monitor Gene Expression A Cautionary TaleKunal KumarОценок пока нет
- Is Rett Syndrome A Loss-Of-Imprinting Disorder?: News and ViewsДокумент2 страницыIs Rett Syndrome A Loss-Of-Imprinting Disorder?: News and ViewsMirabella AstraОценок пока нет
- Lovely Professional University: Assignment ReportДокумент5 страницLovely Professional University: Assignment ReportAdarshОценок пока нет
- Review Polarity Proteins in Axon Specification and SynaptogenesisДокумент14 страницReview Polarity Proteins in Axon Specification and Synaptogenesisrocambolescas perthОценок пока нет
- Transposition of Native Chromatin For Fast and Sensitive Epigenomic Profiling of Open Chromatin, Dna-Binding Proteins and Nucleosome PositionДокумент8 страницTransposition of Native Chromatin For Fast and Sensitive Epigenomic Profiling of Open Chromatin, Dna-Binding Proteins and Nucleosome PositionKito TongHuiОценок пока нет
- Epigenetics in Alternative Pre-mRNA Splicing: ReviewДокумент11 страницEpigenetics in Alternative Pre-mRNA Splicing: ReviewThiagoОценок пока нет
- Strahl and Hamoen (2010) - Membrane PotentialДокумент6 страницStrahl and Hamoen (2010) - Membrane PotentialMaria Dakes StavrakakisОценок пока нет
- POLGДокумент11 страницPOLGİzem DevecioğluОценок пока нет
- RNA Therapeutics Are Stepping Out of The Maze: ForumДокумент4 страницыRNA Therapeutics Are Stepping Out of The Maze: Forum01Syafira Khairunissa MОценок пока нет
- 1995c # SundolДокумент5 страниц1995c # SundolnugrahoneyОценок пока нет
- Embo Embo Embo: A New p38 MAP Kinase-Regulated Transcriptional Coactivator That Stimulates P53-Dependent ApoptosisДокумент12 страницEmbo Embo Embo: A New p38 MAP Kinase-Regulated Transcriptional Coactivator That Stimulates P53-Dependent Apoptosistalha saleemОценок пока нет
- BioorgMedChem 2007 McKay Modelling - BZPДокумент12 страницBioorgMedChem 2007 McKay Modelling - BZPFred DaviersОценок пока нет
- Mechanism of mRNA Transport in The NucleusДокумент6 страницMechanism of mRNA Transport in The NucleusNarayla LailaОценок пока нет
- Williams - 1999 - PKR A Sentinel Kinase For Cellular StressДокумент9 страницWilliams - 1999 - PKR A Sentinel Kinase For Cellular StressGuillem PratsОценок пока нет
- mTOR and MAPK: From Localized Translation Control To EpilepsyДокумент10 страницmTOR and MAPK: From Localized Translation Control To Epilepsysimran ranaОценок пока нет
- Physical Interaction of Delta1, Jagged1, and Jagged2 With Notch1 and Notch3 ReceptorsДокумент5 страницPhysical Interaction of Delta1, Jagged1, and Jagged2 With Notch1 and Notch3 ReceptorsMounikaGoruganthuОценок пока нет
- 1 s2.0 S0006349511027986 Main PDFДокумент1 страница1 s2.0 S0006349511027986 Main PDFDiego TulcanОценок пока нет
- 2009 OjehДокумент13 страниц2009 OjehAtrocitus RedОценок пока нет
- QSB 03 - Chemical Components and Energy2Документ30 страницQSB 03 - Chemical Components and Energy2fta2013Оценок пока нет
- PIIS0092867413002754Документ2 страницыPIIS0092867413002754Arpan NandyОценок пока нет
- 1 gRNAДокумент6 страниц1 gRNA冯博士Оценок пока нет
- 2003-Culliman-PERK and NRF2Документ12 страниц2003-Culliman-PERK and NRF2HaiОценок пока нет
- Bergo 2014Документ16 страницBergo 2014SARAHI MACIAS REALОценок пока нет
- Review: Whole-Cell cAMP and PKA Activity Are Epiphenomena, Nanodomain SignalingДокумент16 страницReview: Whole-Cell cAMP and PKA Activity Are Epiphenomena, Nanodomain SignalingRenee DayОценок пока нет
- EPISSAGE À LIRE Rogalska Et Al. Regulatation of pre-mRNA Splicing. Nat Rev Gen 2022Документ19 страницEPISSAGE À LIRE Rogalska Et Al. Regulatation of pre-mRNA Splicing. Nat Rev Gen 2022Léo VidoniОценок пока нет
- Poro ExocitosisДокумент9 страницPoro ExocitosisCindy GarciaОценок пока нет
- Jogodzik 2018Документ26 страницJogodzik 2018furwithtailsОценок пока нет
- Notch-Activated Signaling Cascade Interacts With Mitochondrial Remodeling Proteins To Regulate Cell SurvivalДокумент6 страницNotch-Activated Signaling Cascade Interacts With Mitochondrial Remodeling Proteins To Regulate Cell SurvivalFarah HaqueОценок пока нет
- N6-Methyladenosine Regulates Rna Abundance of Sars-Cov-2: Cell Discovery December 2021Документ5 страницN6-Methyladenosine Regulates Rna Abundance of Sars-Cov-2: Cell Discovery December 2021Ramona AnaОценок пока нет
- tmp4567 TMPДокумент13 страницtmp4567 TMPFrontiersОценок пока нет
- PShapiro Kinase Regulation 08Документ29 страницPShapiro Kinase Regulation 08uma-chenОценок пока нет
- Kalies 1998 PDFДокумент8 страницKalies 1998 PDFEs BobyyОценок пока нет
- DNA To DNA Transcription Might Exist in Eukaryotic Cells: Open Access Library JournalДокумент4 страницыDNA To DNA Transcription Might Exist in Eukaryotic Cells: Open Access Library JournalVhia Labetubun RenoatОценок пока нет
- Miguel-Arribas A, Et Al. NAR 2021Документ15 страницMiguel-Arribas A, Et Al. NAR 2021AntonОценок пока нет
- Adenylate Kinase Phosphotransfer Communicates Cellular Energetic Signals To ATP-sensitive Potassium ChannelsДокумент6 страницAdenylate Kinase Phosphotransfer Communicates Cellular Energetic Signals To ATP-sensitive Potassium Channelsxoco xocoОценок пока нет
- Phosphorylation of P-Glycoprotein by PKA and PKC Modulates Swelling-Activated CL CurrentsДокумент9 страницPhosphorylation of P-Glycoprotein by PKA and PKC Modulates Swelling-Activated CL CurrentsDr-Dalya ShakirОценок пока нет
- 10.1038@s41576 020 0219 yДокумент1 страница10.1038@s41576 020 0219 yCarlos Meza HernandezОценок пока нет
- ReversPrecipyColoides PDFДокумент8 страницReversPrecipyColoides PDFJonathanОценок пока нет
- Raste Gari 2004Документ10 страницRaste Gari 2004oreamigОценок пока нет
- Estr A1 y A2Документ7 страницEstr A1 y A2JonathanОценок пока нет
- Estr ArchipiélagosДокумент7 страницEstr ArchipiélagosJonathanОценок пока нет
- Morphology of Aggregated Asphaltene Structural ModelsДокумент11 страницMorphology of Aggregated Asphaltene Structural ModelsJonathanОценок пока нет
- Molecular Size and Weight of Asphaltene and Asphaltene Solubility Fractions From Coals, Crude Oils and BitumenДокумент11 страницMolecular Size and Weight of Asphaltene and Asphaltene Solubility Fractions From Coals, Crude Oils and BitumenJonathanОценок пока нет
- Ef 050430 GДокумент7 страницEf 050430 GJonathanОценок пока нет
- Geoffrey C. Klein, Sunghwan Kim, Ryan P. Rodgers, and Alan G. MarshallДокумент8 страницGeoffrey C. Klein, Sunghwan Kim, Ryan P. Rodgers, and Alan G. MarshallJonathanОценок пока нет
- Rheology of Asphaltene-Toluene/Water Interfaces: Danuta M. Sztukowski and Harvey W. YarrantonДокумент8 страницRheology of Asphaltene-Toluene/Water Interfaces: Danuta M. Sztukowski and Harvey W. YarrantonJonathanОценок пока нет
- Acevedo S, Ranaudo M, Garcia C, Castillo JДокумент5 страницAcevedo S, Ranaudo M, Garcia C, Castillo JJonathanОценок пока нет
- La 048640 TДокумент9 страницLa 048640 TJonathanОценок пока нет
- Ef0501243 PDFДокумент4 страницыEf0501243 PDFJonathanОценок пока нет
- Zeta Potential and Langmuir Films of Asphaltene Polar FractionsДокумент7 страницZeta Potential and Langmuir Films of Asphaltene Polar FractionsJonathanОценок пока нет
- Jovan OvДокумент7 страницJovan OvJonathanОценок пока нет
- Geoffrey C. Klein, Sunghwan Kim, Ryan P. Rodgers, and Alan G. MarshallДокумент8 страницGeoffrey C. Klein, Sunghwan Kim, Ryan P. Rodgers, and Alan G. MarshallJonathanОценок пока нет
- Determination of Asphalt Molecular Weight Distributions by Gel Permeation ChromatographyДокумент5 страницDetermination of Asphalt Molecular Weight Distributions by Gel Permeation ChromatographyJonathanОценок пока нет
- Asphaltenes in Oil Reservior Recovery: ReviewsДокумент6 страницAsphaltenes in Oil Reservior Recovery: ReviewsKadirov AlgeriaОценок пока нет
- 5 Work and Money: Working To Live, or Living To Work?Документ8 страниц5 Work and Money: Working To Live, or Living To Work?JonathanОценок пока нет
- Ef030030y PDFДокумент7 страницEf030030y PDFJonathanОценок пока нет
- Thermodynamic Micellization Model Asphaltene Precipitation From Petroleum FluidsДокумент12 страницThermodynamic Micellization Model Asphaltene Precipitation From Petroleum FluidsJonathanОценок пока нет
- The Characterization of Asphaltenes by H NMR Relaxation Method: Microsecond Range of Spin-Spin Relaxation TimesДокумент9 страницThe Characterization of Asphaltenes by H NMR Relaxation Method: Microsecond Range of Spin-Spin Relaxation TimesJonathanОценок пока нет
- Effects of Petroleum Resins On Asphaltene Aggregation and Water-In-Oil Emulsion FormationДокумент19 страницEffects of Petroleum Resins On Asphaltene Aggregation and Water-In-Oil Emulsion FormationJonathanОценок пока нет
- By Vojo Deretic and Daniel J. Klionsky: BiologyДокумент8 страницBy Vojo Deretic and Daniel J. Klionsky: BiologyJonathanОценок пока нет
- 7 The 20th Century: ChangesДокумент8 страниц7 The 20th Century: ChangesJonathanОценок пока нет
- 6 Keeping Up With Technology: Developing The AutomobileДокумент8 страниц6 Keeping Up With Technology: Developing The AutomobileJonathanОценок пока нет
- 7 The 20th Century: ChangesДокумент8 страниц7 The 20th Century: ChangesJonathanОценок пока нет
- 5 Stages of Life: Learning To Be HumanДокумент8 страниц5 Stages of Life: Learning To Be HumanJonathanОценок пока нет
- TB4 Unit 2Документ8 страницTB4 Unit 2JonathanОценок пока нет
- Bacteriological Analytical Manual Chapter 21A Examination of Canned FoodsДокумент26 страницBacteriological Analytical Manual Chapter 21A Examination of Canned FoodsFrancis Dave FloresОценок пока нет
- 11list2 23Документ1 страница11list2 23yemocip111Оценок пока нет
- Nikola Saraginovski, Marjan Kiprijanovski, Tosho Arsov, Slavko GeorgievskiДокумент1 страницаNikola Saraginovski, Marjan Kiprijanovski, Tosho Arsov, Slavko GeorgievskiMirko PetrićОценок пока нет
- Select Readings InterДокумент23 страницыSelect Readings Interphuonganh nguyenОценок пока нет
- Biology Answers PDFДокумент30 страницBiology Answers PDFأسيل الشلةОценок пока нет
- AMINOGLYCOSIDESДокумент2 страницыAMINOGLYCOSIDESDiana Marcela Gomez PintoОценок пока нет
- Kalindee S. Shinde and S. G. BorkarДокумент6 страницKalindee S. Shinde and S. G. BorkarKalindee SОценок пока нет
- Bioinformatics PDFДокумент336 страницBioinformatics PDFJoel CordeiroОценок пока нет
- "Omul" The FishДокумент3 страницы"Omul" The FishIoana Estela PătrulescuОценок пока нет
- Cycd. Jumbo Puff or Cycd. Taiwan GoldДокумент14 страницCycd. Jumbo Puff or Cycd. Taiwan GoldMick TalbotОценок пока нет
- Contoh CV Bahasa Inggris BlogbintangДокумент26 страницContoh CV Bahasa Inggris BlogbintangFirman Juniardi PutraОценок пока нет
- Basic Medical Sciences (3rd Ed)Документ442 страницыBasic Medical Sciences (3rd Ed)aeyousefОценок пока нет
- Acute Pharyngitis in Children and Adolescents - Symptomatic Treatment UpToDateДокумент10 страницAcute Pharyngitis in Children and Adolescents - Symptomatic Treatment UpToDateAaron VargasОценок пока нет
- Inotozumab OzogamicinДокумент14 страницInotozumab OzogamicinTaraОценок пока нет
- Bone DevelopmentДокумент25 страницBone DevelopmentZeyОценок пока нет
- Chapter One A CellДокумент46 страницChapter One A CellLeon MarkoОценок пока нет
- Vitex Agnus Castus Molecular Marker Compounds Extraction and Optimization Using HPLCДокумент7 страницVitex Agnus Castus Molecular Marker Compounds Extraction and Optimization Using HPLCDr. Varaprasad BobbaralaОценок пока нет
- PHOTOSYNTESISДокумент23 страницыPHOTOSYNTESISJustine Kate PurisimaОценок пока нет
- Concentration of Methanol and Ethanol in Alcoholic Drink by GCДокумент10 страницConcentration of Methanol and Ethanol in Alcoholic Drink by GCYunus HidayatОценок пока нет
- 2010 National Report On Sustainable ForestsДокумент214 страниц2010 National Report On Sustainable ForestsLakeCoNewsОценок пока нет
- The Respiratory System: Lecture Presentation by Patty Bostwick-Taylor Florence-Darlington Technical CollegeДокумент30 страницThe Respiratory System: Lecture Presentation by Patty Bostwick-Taylor Florence-Darlington Technical CollegeFakultas Kedokteran UnhanОценок пока нет
- 2010 Nototriton Tomamorum TexiguatДокумент17 страниц2010 Nototriton Tomamorum TexiguatJosue Ramos GaldamezОценок пока нет
- NutrigenomicsДокумент8 страницNutrigenomicsGabriel MarcosОценок пока нет
- Discuss Evolutionary Explanations For Partner Preferences PLANДокумент3 страницыDiscuss Evolutionary Explanations For Partner Preferences PLANShamia FioreОценок пока нет
- Etymology: Meros Meaning "Part". The Term Was Coined in 1833 by Jöns Jacob Berzelius, Although HisДокумент12 страницEtymology: Meros Meaning "Part". The Term Was Coined in 1833 by Jöns Jacob Berzelius, Although HisZoya KapoorОценок пока нет
- Chapter Three Thermal Comfort and Health FS2023-24Документ27 страницChapter Three Thermal Comfort and Health FS2023-24hssan AliОценок пока нет