Академический Документы
Профессиональный Документы
Культура Документы
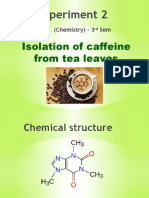
Experiment 1 Isolation of Caffeine From A Tea Bag
Загружено:
Nur AthirahОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Experiment 1 Isolation of Caffeine From A Tea Bag
Загружено:
Nur AthirahАвторское право:
Доступные форматы
Instruction sheet for students.
CHM556
Experiment 1
Isolation of Caffeine from a Tea Bag
For background of this experiment, refer to experiment 11B, page 498 of the
laboratory textbook: Introduction to Organic Laboratory Techniques, A Small Scale
Approach, 3rd Edition by Engel, Kriz, Lapman and Pavia.
In this experiment, you will extract caffeine from tea bag. This is an example of
isolation of a natural product utilizing acid base extraction technique. Read
introductory part of experiment 11 carefully before proceeding to the actual procedure
in experiment 11B.
Please review technique on Separation and Purification Scheme (4; pg 461) including
Extractions with Separating Funnel (4A; pg 462).
Conduct the experiment as described in the laboratory text book with the following
modifications.
1. Use a 100 ml beaker and 50 ml of water to make the tea solution.
2. Transfer the hot tea solution into a small conical flask. Then add 0.5 g of
sodium carbonate.
3. Then filter the solution into a small separatory funnel. Use cotton for
filtration not filter paper.
4. Add 15 ml of methylene chloride to the separatory funnel and start the
extraction. You will need to wait for the emulsion to break.
5. Drain off the lower methylene chloride layer and add 10 ml of fresh
methylene chloride. (Repeat this step 2 times).
6. Dry the combined methylene chloride layer using anhydrous sodium
sulphate.
7. Transfer the dried methylene chloride solution into a pre-weighed small
round bottomed flask and evaporate off the solvent using the rotary-
evaporator.
Your report for this experiment should include:
a) The extraction scheme.
b) Percentage of caffeine in the tea.
Organic Chemistry Section,
Center of Chemistry and Environmental Studies,
Faculty of Applied Sciences,
Universiti Teknologi MARA.
Вам также может понравиться
- Activity 10 Extraction and Recrystallization of Caffeine From Tea ProcedureДокумент5 страницActivity 10 Extraction and Recrystallization of Caffeine From Tea Procedurejessie jacolОценок пока нет
- LABORATORY MANUAL FOR A MINI PROJECT: MSCB 1113 BIOCHEMISTRY & MICROBIAL PHYSIOLOGYОт EverandLABORATORY MANUAL FOR A MINI PROJECT: MSCB 1113 BIOCHEMISTRY & MICROBIAL PHYSIOLOGYОценок пока нет
- Experimental Teaching Reform and Practice On ExtraДокумент6 страницExperimental Teaching Reform and Practice On ExtraNguyễn Xuân TùngОценок пока нет
- Isolation of Caffeine From A Tea BagДокумент4 страницыIsolation of Caffeine From A Tea BagohhiОценок пока нет
- Somatic Embryogenesis for Micropropagation of Coconut (Cocos nucifera L.)От EverandSomatic Embryogenesis for Micropropagation of Coconut (Cocos nucifera L.)Оценок пока нет
- EXPERIMENT 1 Isolation of CaffeineДокумент7 страницEXPERIMENT 1 Isolation of CaffeineNajwa ZulkifliОценок пока нет
- Introductory Microbiology Lab Skills and Techniques in Food ScienceОт EverandIntroductory Microbiology Lab Skills and Techniques in Food ScienceОценок пока нет
- 7-Extraction and Recrystallization of Caffeine From Tea (P)Документ5 страниц7-Extraction and Recrystallization of Caffeine From Tea (P)Gezem GigantoОценок пока нет
- Lab #3 "The Separation and Isolation of Caffeine From Tea by Extraction With Hot Water"Документ5 страницLab #3 "The Separation and Isolation of Caffeine From Tea by Extraction With Hot Water"api-281675422Оценок пока нет
- Caffeine ExperimentДокумент6 страницCaffeine ExperimentHolyZikrОценок пока нет
- Caffeine Extraction (WU) Rev 1-2015Документ5 страницCaffeine Extraction (WU) Rev 1-2015Santpal KalraОценок пока нет
- Natural Product Isolation CaffeineДокумент5 страницNatural Product Isolation CaffeinelaughinnNahgaОценок пока нет
- Formal Report ExtractionДокумент5 страницFormal Report ExtractionPhilina PasicolanОценок пока нет
- Protocolo de Extracción de ADN Bacteriano - AusubelДокумент5 страницProtocolo de Extracción de ADN Bacteriano - AusubelLesly CastilloОценок пока нет
- Isolation of Caffeine ExperimentДокумент8 страницIsolation of Caffeine ExperimentdikshaОценок пока нет
- Beta Carotene AnalysisДокумент5 страницBeta Carotene AnalysisChandra Shekhar BОценок пока нет
- Experiment 3Документ3 страницыExperiment 3BuiHopeОценок пока нет
- Lab Manual Micro Biotech DraftДокумент10 страницLab Manual Micro Biotech Drafthồng Phúc Tôn NguyễnОценок пока нет
- Exp 1Документ5 страницExp 1afiqahОценок пока нет
- Extraction of Caffeine From TeaДокумент7 страницExtraction of Caffeine From Teaامنة محمد حسن احمدОценок пока нет
- Solvent Extraction of Caffeine From TeaДокумент4 страницыSolvent Extraction of Caffeine From TeaJocelyn Sun100% (1)
- Isolation of CaffeineДокумент3 страницыIsolation of CaffeineDaniel McDermott0% (1)
- Preparation of 4-Methylcyclohexene From Dehydration of 4-MethylcyclohexanolДокумент8 страницPreparation of 4-Methylcyclohexene From Dehydration of 4-MethylcyclohexanolHidayu AdnanОценок пока нет
- UVD C C: Etermination OF Affeine OntentДокумент3 страницыUVD C C: Etermination OF Affeine Ontentlox agencyОценок пока нет
- VanillinДокумент14 страницVanillinReksy WibowoОценок пока нет
- CHM 457 Exp 2Документ9 страницCHM 457 Exp 2Ayish MataОценок пока нет
- S Determination of Caffeine in BeveragesДокумент5 страницS Determination of Caffeine in BeveragesVioleta Grigoras100% (1)
- CBSE Chemistry Project Class 12Документ10 страницCBSE Chemistry Project Class 12SachithОценок пока нет
- Ibuprofen in WerkingДокумент18 страницIbuprofen in WerkingMatthias LamoteОценок пока нет
- Exp10 PDFДокумент3 страницыExp10 PDFعمر العنزيОценок пока нет
- Experiment 21 Caffeine ColaДокумент3 страницыExperiment 21 Caffeine ColaJosef Franz CruzОценок пока нет
- Isolation of Caffeine From Tea: Experiment 18Документ7 страницIsolation of Caffeine From Tea: Experiment 18Murugan MОценок пока нет
- Extraction of Caffeine From Coffee and Tea: Organized by Lecturers: Sharifa A. Al-Ghamdi& Maha BaljoonДокумент4 страницыExtraction of Caffeine From Coffee and Tea: Organized by Lecturers: Sharifa A. Al-Ghamdi& Maha BaljoonĐoàn NgọcОценок пока нет
- Caffeine Extraction: Lab ReportДокумент7 страницCaffeine Extraction: Lab Reportapi-409656379Оценок пока нет
- Extracting Caffeine Experiment GuideДокумент7 страницExtracting Caffeine Experiment GuideJovennОценок пока нет
- The Extraction of Caffeine From TeaДокумент18 страницThe Extraction of Caffeine From Teaapi-255504065100% (1)
- Single ExtractionДокумент3 страницыSingle Extractioncarlyzza021412Оценок пока нет
- Project On Caffeine Content in Tea ExperimentДокумент12 страницProject On Caffeine Content in Tea ExperimentAjay RajputОценок пока нет
- Mol Bio Dna ProtocolДокумент3 страницыMol Bio Dna ProtocolArianne ManuelОценок пока нет
- Lab Report Experiment 2 CHM457Документ9 страницLab Report Experiment 2 CHM457lala lalaОценок пока нет
- Chemistry Project ON Determine Caffine in Different Tea SamplesДокумент16 страницChemistry Project ON Determine Caffine in Different Tea SamplesAbhishek Tiwari100% (1)
- Isolation of Caffeine From A Tea BagДокумент9 страницIsolation of Caffeine From A Tea Baginsyirah shazrinОценок пока нет
- Extraction and Recrystallization of CaffeineДокумент3 страницыExtraction and Recrystallization of CaffeineTommy BetyОценок пока нет
- Amit ChemДокумент18 страницAmit ChemommarabindajenaОценок пока нет
- Mammalian Genomic DNA Miniprep KitsДокумент6 страницMammalian Genomic DNA Miniprep KitsRajan RawalОценок пока нет
- Data and Results: Table 1: Caffeine Extraction Recovery DataДокумент4 страницыData and Results: Table 1: Caffeine Extraction Recovery DataKarl CarandangОценок пока нет
- Caffeine Extraction From TeaДокумент4 страницыCaffeine Extraction From Teaahmadshakifi89Оценок пока нет
- 4) DNA ExtractionДокумент11 страниц4) DNA ExtractionajiesyahbarieОценок пока нет
- Phys Chem 3 Test 1 2013Документ30 страницPhys Chem 3 Test 1 2013Clement ThabangОценок пока нет
- Caffeine PDFДокумент9 страницCaffeine PDFAmitAgarwalОценок пока нет
- LONG CAFFEINE UVLong Caffeine UvДокумент8 страницLONG CAFFEINE UVLong Caffeine UvzaОценок пока нет
- UV Determination of Caffeine in SodaДокумент3 страницыUV Determination of Caffeine in SodaMArs NguyễnОценок пока нет
- Recrystallization and Melting Point Determination of Benzoic AcidДокумент6 страницRecrystallization and Melting Point Determination of Benzoic AcidAnonymous GO6JVW9Wud0% (1)
- Extraction of Catteine in Tea LeavesДокумент8 страницExtraction of Catteine in Tea LeavesShanaz ShaxawanОценок пока нет
- DNA Extraction From Fungi, Yeast, and BacteriaДокумент2 страницыDNA Extraction From Fungi, Yeast, and Bacteriavishankgupta100% (1)
- CHM556 Experiment 1Документ6 страницCHM556 Experiment 1nurulfatinizzatyОценок пока нет
- Laboratory Report 2 - Canillas, CДокумент5 страницLaboratory Report 2 - Canillas, CColline Faye CanillasОценок пока нет
- Crystallization S16Документ4 страницыCrystallization S16Muhammad Ayan MalikОценок пока нет
- Lab 3Документ7 страницLab 3Nur AthirahОценок пока нет
- chm432 - 4Документ2 страницыchm432 - 4Nur AthirahОценок пока нет
- 1Документ2 страницы1Nur AthirahОценок пока нет
- 1Документ2 страницы1Nur AthirahОценок пока нет
- chm457 2Документ3 страницыchm457 2Nur AthirahОценок пока нет
- chm457 4Документ3 страницыchm457 4Nur Athirah0% (1)
- Chm421 LabДокумент3 страницыChm421 LabNur AthirahОценок пока нет
- ResultsДокумент2 страницыResultsNur AthirahОценок пока нет
- Elc650 Email GuidelinesДокумент2 страницыElc650 Email GuidelinesNur Athirah50% (2)
- References: Mistry - (Smith) /chapter - 24: - Carbonyl - Condensation - Reactions/24.8: - The - Michael - ReactionДокумент2 страницыReferences: Mistry - (Smith) /chapter - 24: - Carbonyl - Condensation - Reactions/24.8: - The - Michael - ReactionNur AthirahОценок пока нет
- Experiment 5: Preparation of An, Unsaturated Ketone Via Michael Addition and Aldol Condensation ReactionsДокумент10 страницExperiment 5: Preparation of An, Unsaturated Ketone Via Michael Addition and Aldol Condensation ReactionsNur AthirahОценок пока нет
- Inorganic Chemistry: Experiment 7: Measurement of Physical Properties and Isomerism of ComplexesДокумент4 страницыInorganic Chemistry: Experiment 7: Measurement of Physical Properties and Isomerism of ComplexesNur AthirahОценок пока нет
- Exp 1Документ5 страницExp 1Nur AthirahОценок пока нет
- Title: Preparation of 4-MethylcyclohexeneДокумент7 страницTitle: Preparation of 4-MethylcyclohexeneNur AthirahОценок пока нет
- KRESMAДокумент2 страницыKRESMANur AthirahОценок пока нет
- LOLДокумент1 страницаLOLNur AthirahОценок пока нет
- Cocl 6 NH Orange-Yellow Cocl 5 NH H O Red Cocl 5 NH Purple Cocl 4 NH GreenДокумент3 страницыCocl 6 NH Orange-Yellow Cocl 5 NH H O Red Cocl 5 NH Purple Cocl 4 NH GreenNur AthirahОценок пока нет
- ExpДокумент6 страницExpNur AthirahОценок пока нет
- 2Документ2 страницы2Nur AthirahОценок пока нет
- Obesity: Term Paper OnДокумент13 страницObesity: Term Paper OnNur AthirahОценок пока нет
- OBESITYДокумент2 страницыOBESITYNur AthirahОценок пока нет
- Potassium TrihydrateДокумент5 страницPotassium TrihydrateTeresa Davis60% (15)
- Obesity: Term Paper OnДокумент13 страницObesity: Term Paper OnNur AthirahОценок пока нет
- Exp 6 BioДокумент1 страницаExp 6 BioNur AthirahОценок пока нет
- Exp 5 BioДокумент1 страницаExp 5 BioNur AthirahОценок пока нет
- OBESITYДокумент2 страницыOBESITYNur AthirahОценок пока нет
- Exp 5 BioДокумент1 страницаExp 5 BioNur AthirahОценок пока нет
- 1.0 Chemical BondingДокумент42 страницы1.0 Chemical BondingNur AthirahОценок пока нет
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincОт EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincРейтинг: 3.5 из 5 звезд3.5/5 (137)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolОт EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolОценок пока нет
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeОт EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeРейтинг: 5 из 5 звезд5/5 (4)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeОт EverandChemistry for Breakfast: The Amazing Science of Everyday LifeРейтинг: 4.5 из 5 звезд4.5/5 (90)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsОт EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsОценок пока нет
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideОт EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideОценок пока нет
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactОт EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactРейтинг: 5 из 5 звезд5/5 (5)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeОт EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeРейтинг: 4 из 5 звезд4/5 (1)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsОт EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsРейтинг: 4 из 5 звезд4/5 (146)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeОт EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeРейтинг: 5 из 5 звезд5/5 (1)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeОт EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeОценок пока нет
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsОт EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsРейтинг: 5 из 5 звезд5/5 (3)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableОт EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableРейтинг: 3.5 из 5 звезд3.5/5 (22)
- Taste: Surprising Stories and Science About Why Food Tastes GoodОт EverandTaste: Surprising Stories and Science About Why Food Tastes GoodРейтинг: 3 из 5 звезд3/5 (20)
- The Periodic Table: A Very Short IntroductionОт EverandThe Periodic Table: A Very Short IntroductionРейтинг: 4.5 из 5 звезд4.5/5 (3)
- Tribology: Friction and Wear of Engineering MaterialsОт EverandTribology: Friction and Wear of Engineering MaterialsРейтинг: 5 из 5 звезд5/5 (1)
- Ingredients: A Visual Exploration of 75 Additives & 25 Food ProductsОт EverandIngredients: A Visual Exploration of 75 Additives & 25 Food ProductsРейтинг: 4 из 5 звезд4/5 (1)
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookОт EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookОценок пока нет
- Chemistry for Breakfast: The Amazing Science of Everyday LifeОт EverandChemistry for Breakfast: The Amazing Science of Everyday LifeРейтинг: 4.5 из 5 звезд4.5/5 (14)
- High School Chemistry: Comprehensive Content for High School ChemistryОт EverandHigh School Chemistry: Comprehensive Content for High School ChemistryОценок пока нет
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactОт EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactРейтинг: 5 из 5 звезд5/5 (1)
- Chemistry: a QuickStudy Laminated Reference GuideОт EverandChemistry: a QuickStudy Laminated Reference GuideРейтинг: 5 из 5 звезд5/5 (1)