Академический Документы
Профессиональный Документы
Культура Документы
Isomerism DPP
Загружено:
RAGHUL MИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Isomerism DPP
Загружено:
RAGHUL MАвторское право:
Доступные форматы
Work sheet on KVPY FIITJEE
Bangalore CENTRE
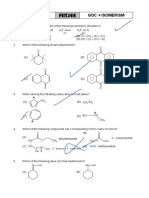
Subject: CHEMISTRY Topic : Hydrocarbon and GOC Date of Distribution :
NAME: BATCH : Max. Marks :
ONE OR MORE THAN ONE CORRECT TYPE
1. Which of the following preparation method will be better than others in terms of yield of n-butane?
N a , D .E . O
(A) CH3CH2 Br (B) e le c tr o ly s is
CH3CH2
D - +
O Na
( i) M g , D .E . O
(C) CH3CH2CH2CH2 Br (D)
( ii) H 2 O Zn + H3C C OH
CH3CH2 Br
2. In which of the following reactions the major product will have chiral carbon(s)?
D 2 / Ni B r2, h n
(A) CH3CH2 CH2 (B) H3C CH2 CH2 CH3
M o n o b r o m in a t io n
Et O
Z n - C u + E tO H Zn - H g + conc. H C l
(C)
H3C C CH2 Br H3C C CH CH2CH3
(D)
CH2CH2CH3 CH3
3. Among different isomers of pentane; which of the following mentioned properties have been arranged in
correct order ?
(A) n-pentane > iso-pentane> neo-pentane (boiling point)
(B) neo-pentane > n-pentane > iso-pentane (melting point)
(C) n-pentane > iso-pentane > neo-pentane (heat of combustion)
(D) neo-pentane > iso-pentane > neo-pentane (rate of reaction towards Br 2, hn)
4. 1 mole of aqueous solution of sodium acetate is electrolysed completely. What is the total volume of gases
evolves at STP
(A) 22.4 L (B) 44.8 L (C) 67.2 L (D) 33.6 L
1 2
H CH3
H
B r.
5. 3 Relative ease of abstraction of H by Br . follows the order
H
H3C
H3C
(A) 1 > 2 > 3 (B) 2 > 3 > 1 (C) 3 > 1 > 2 (D) 3 > 2 > 1
CH3
B r2; h n F r a c tio n a l
6. H3C X Y fractions ; X & Y respectively are
M o n o b r o m in a tio n isomers d i s t i l l a t i o n
CH3
(A) 8, 5 (B) 8, 8 (C) 8, 4 (D) None of these
7. Which of the following carboxylic acid can be decarboxylated most easily using soda-lime?
O O O O
(A) (B) (C) (D)
C C C C
OH OH OH OH
8. CH3 – CH2 – CH3 can be prepared by
L iA lH 4
( i) M g , D E
(A) H3C CH CH3 (B) H3C CH CH3
Br ( ii) N H 3
Br
FIITJEE Ltd. Bangalore Centre www.fiitjee.com
Work sheet Topic : Page 2 of 4
O - O
N 2H + O H R ed P + H I
(C) H3C C CH3
4
(D)
CH3CH2 C H
9. How many dicarbonyls (excludinig stereoisomerism) with molecular formula C 5H8O2 upon reduction with
N2H4 + OH - will form an unbranched alkane with molecular formula C5H12
(A) 6 (B) 7 (C) 8 (D) 9
10. Which of the following alkenes will have heat of hydrogenation more than that of others?
(A) (B) (C) (D) All have same value
11. 0.037 g of an alcohol, R–OH was added to CH 3MgI and the gas evolved measured 11.2 cm 3 at STP. What is
the molecular mass of R–OH ?
(A) 46 (B) 60 (C) 74 (D) 88
12.
(I) (II) (III)
The correct order of reactivity of I, II & III towards addition reactions is :
(A) I > III > II (B) I > II > III (C) III > II > I (D) III > I > II
13. Observe the given compounds and answer the following questions.
C O 2H C O 2H C O 2H C O 2H C O 2H
H O H H O H H O H H O H H O H
H O H H O H H O H H O H H O H
H O H H O H H O H H O H H O H
C O 2H C O 2H C O 2 H C O 2H C O 2H
(I) (II) (III) (IV ) (V )
(i) Which of the above formulae represent identical compounds?
(a) I and II (b) I and IV (c) II and IV (d) III and IV
(ii) Which of the above compounds are enantiomers?
(a) II and III (b) III and IV (c) III and V (d) I and V
14. What observed rotation is expected when a 1.5 M solution of (R)-2-butanol is mixed with an equal volume of a
0.75 M solution of racemic 2-butanol and the resulting solution is analysed in a sample container that is 1 dm
long? The specific rotation of (R)-2-butanol is -13.9 0 ml gm–1 dm–1.
(a) +0.770 (b) – 0.770 (c) +0.350 (d) – 0.350
15. A pure sample of 2-chloro butane shows rotation of PPL by 300 in standard conditions. When above sample is
made impure by mixing its opposite form, so that the composition of the mixture becomes 87.5% d-form and
12.5% l-form, then what will be observed rotation for the mixture
(a) -22.50 (b) +22.50 (c) +7.50 (d) -7.50
16. Isomers which can be interconverted through rotation around a single bond are
(a) Conformers (b) Diastereomers (c) Enantiomers (d) Positional isomers
H 3C H
C == C H
17. The H 3C C shows
CO O H
CH 3
(a) Geometrical isomerism (b) Optical isomerism
(c) Geometrical and optical isomerism (d) Tautomerism
18. How many optically active stereoisomers are possible for butane-2, 3-diol?
(a) 1 (b) 2 (c) 3 (d) 4
19. The number of possible enantiomeric pairs that can be produced during monochlorination of
2-methyl butane is
(a) 2 (b) 3 (c) 4 (d) 1
20. When cyclohexane is poured on water, it floats, because
FIITJEE Ltd. Bangalore Centre www.fiitjee.com
Work sheet Topic : Page 3 of 4
(a) Cyclohexane is in ‘boat’ form (b) Cyclohexane is in ‘chair’ form
(c) Cyclohexane is in ‘crown’ form (d) Cyclohexane is less dense than water
21. Which of the following compounds will show geometrical isomerism?
(a) 2-butene (b) propene (c) 1-phenylpropene (d) 2-methyl-2-butene
22. Which of the following compound will exhibit geometrical isomerism?
(a) 1-Phenyl-2-butene (b) 3-Phenyl-1-butene
(c) 2-Phenyl-1-butene (d) 2-methyl-2-butene
23. The number of isomers for the compound with molecular formula C 2BrClFI is
(a) 3 (b) 4 (c) 5 (d) 6
24. Which of the following compounds exhibits optical isomerism?
(a) 2-methylbutene-1 (b) 3-methylbutyne-1
(c) 3-methylbutanoic acid (d) 2-methylbutanoic acid
4
25. In the given conformation, if C2 is rotated about C2—C3 bond anticlockwise by an angle of CH 3
1200 then the conformation obtained is H H
(a) Fully eclipsed conformation
(b) Partially eclipsed conformation
3
(c) Gauche conformation 2
(d) Staggered conformation
26. On monochlorination of 2-methylbutane, the total number of chiral compounds formed is H H
(a) 2 (b) 4 (c) 6 (d) 8 1 CH 3
27. The number of structural isomers for C6H14 is
(a) 3 (b) 4 (c) 5 (d) 6
28. Statement-1 : Molecules that are not superimposable on their mirror images are chiral.
Because
Statement-2 : All chiral molecules have chiral contres.
(a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1
(b) Statement-1 is True; Statement-2 is True; Statement-2 is not correct explanation for Statement-1
(c) Statement-1 is True, Statement-2 is False
(d) Statement-1 is False, Statement-2 is True.
29. The correct statement(s) concerning the structures E, F and G is(are)
O O H H 3C CH 3
H C3
H 3C
H 3C CH 3 H 3C CH 3 H 3C O H
(E ) (F ) (G )
(a) E, F and G are resonance structures (b) E, F and E, G are tautomers
(c) F and G are geometrical isomers (d) F and G are diastereomers
SUBJECTIVE
30. Write structural formulae for all the isomeric alcohols having the molecular formula C 4H10O. (4)
31. Identify the pairs of enantiomers and diastereomers from the following compound I, II and III.
CH 3 CH 3 CH 3
H O H O H H O H H
O H H O H H H O H
CH 3 CH 3 CH 3
(I) (II) (III)
(Enantiomers-I and III; diastereomers-I & II and II & III )
32. What are the relationship between the following pairs of isomers?
Br C O 2H Br C O 2H Br Br
(A) (B) Br Br
and and
FIITJEE Ltd. Bangalore Centre www.fiitjee.com
Work sheet Topic : Page 4 of 4
CH O CH O
CH O C H 2O H
H O H H O H
H N H 2 H O H
(C) H O H and H O H (D) H O H and H N H
H O H H O H 2
C H 2O H CH O
C H 2O H C H 2O H
H C l
H H H CH 3
(E) and (F) and
C l Ph H Ph
CH 3 H
(A) Geometrical isomers & Diastereomers (B) Positional (C) Optical (Diastereomers)
(D) Diastereomers (E) Enantiomers (F) Geometrical isomers (Diastereomers)
33. Calculate the total number of geometrical isomers possible for
H C H =C H –C H 3
(A) (B) C == C == C
Cl C H =C H –C H 3
(A) 16 (B) 4
34. With reasons, state whether each of the following compounds I to IX is chiral.
Cl C l
(I) H (II) H (III)
Cl H
H O 2C C O 2H
H C l
Ph
Cl C O 2H O O M e
(IV) C == C == C (V) (VI) (VII) S == O
Cl M e Ph
C O 2H
achiral : I, III, IV ; chiral : II, V, VI, VII
Comprehension
For the given question (1, 2, 3) consider the following reaction .
CH3
light monohalogenation product
X2
35. Light is involved in which step of the reaction :
(A) Initiation only (B) Termination only
(C) Propagation only (D) Propagation and Termination
36. Which halogen will give the best yield of a single monohalogenation product ?
(A) F2 (B) Cl2 (C) Br2 (D) I2
37. How many monohalo derivatives are possible (excluding stereoisomes) ?
(A) 3 (B) 4 (C) 5 (D) 6
FIITJEE Ltd. Bangalore Centre www.fiitjee.com
Вам также может понравиться
- 1 Mechanics: Cbse Neet JEE Mains AdvancedДокумент11 страниц1 Mechanics: Cbse Neet JEE Mains Advancedvsrajkumar100% (6)
- GRB Physics For Competitions Vol 1Документ853 страницыGRB Physics For Competitions Vol 1Shubham Gupta85% (20)
- Chapter36 - BiomoleculesДокумент19 страницChapter36 - BiomoleculesAkash GoelОценок пока нет
- Reduction, Oxidation - Hydrolysis APSP PDFДокумент24 страницыReduction, Oxidation - Hydrolysis APSP PDFGOURISH AGRAWALОценок пока нет
- Reduction, Oxidation - Hydrolysis Exercise PDFДокумент24 страницыReduction, Oxidation - Hydrolysis Exercise PDFGOURISH AGRAWAL100% (3)
- Carbocation RearrangementДокумент4 страницыCarbocation RearrangementManas J. AggarwalОценок пока нет
- BATCH XI, XII & DROPPER’S TOPIC STEREOISOMERISM DPP 13Документ43 страницыBATCH XI, XII & DROPPER’S TOPIC STEREOISOMERISM DPP 13arryan keshanОценок пока нет
- Goc Worksheet of EtoosДокумент30 страницGoc Worksheet of EtoosOmendra SinghОценок пока нет
- Goc + IsomerismДокумент5 страницGoc + IsomerismRohail HussainОценок пока нет
- Halogen Derivatives PDFДокумент38 страницHalogen Derivatives PDFastha100% (1)
- Organic Chemistry Revision for JEE AdvancedДокумент35 страницOrganic Chemistry Revision for JEE AdvancedSubhrota PradhanОценок пока нет
- GOCДокумент15 страницGOCprashant sharmaОценок пока нет
- IIT-JEE Chemistry by N.J. sir: Aldehydes and KetonesДокумент10 страницIIT-JEE Chemistry by N.J. sir: Aldehydes and KetonesMahendra ChouhanОценок пока нет
- Qualitative Analysis - DPP'sДокумент15 страницQualitative Analysis - DPP'sVanshaj GuptaОценок пока нет
- Reaction IntermediatesДокумент32 страницыReaction Intermediatestechno studioОценок пока нет
- A - 1 (Isomerism, Reaction Mechanism) - Question PaperДокумент11 страницA - 1 (Isomerism, Reaction Mechanism) - Question PaperSachin DedhiaОценок пока нет
- Isomerism: IndexДокумент38 страницIsomerism: IndexDEV UPPALОценок пока нет
- Jitendra Hirwani: Problem Solving Techniques of Physical Chemistry For NeetДокумент18 страницJitendra Hirwani: Problem Solving Techniques of Physical Chemistry For NeetabhishekОценок пока нет
- Hydrocarbon Level I KVPYДокумент2 страницыHydrocarbon Level I KVPYRAGHUL MОценок пока нет
- Hydrocarbon Level I KVPYДокумент2 страницыHydrocarbon Level I KVPYRAGHUL MОценок пока нет
- Alcohol, Ether & Phenol - QuestionДокумент3 страницыAlcohol, Ether & Phenol - Questionbest badmintonОценок пока нет
- Class XII Computer Project - Hotel ManagementДокумент29 страницClass XII Computer Project - Hotel ManagementLakshmi Puthiyedath71% (7)
- Nurturing Organic Chemistry KnowledgeДокумент29 страницNurturing Organic Chemistry KnowledgeTRUPTIRANI PUROHIT50% (2)
- Isomerism DPP PDFДокумент69 страницIsomerism DPP PDFfootball 1063150% (2)
- Carboxylic Acid and Amines Worksheet PDFДокумент22 страницыCarboxylic Acid and Amines Worksheet PDFd anjilappaОценок пока нет
- Isomerism Sheet - by NJ Sir PDFДокумент42 страницыIsomerism Sheet - by NJ Sir PDFVikas Rana100% (1)
- PHYSICS O-LEVEL PAST PAPER QUESTIONS ON MOMENTSДокумент7 страницPHYSICS O-LEVEL PAST PAPER QUESTIONS ON MOMENTSelty TanОценок пока нет
- NEET JH SIR DPP Exercise Chemical BondingДокумент19 страницNEET JH SIR DPP Exercise Chemical BondingSunnyОценок пока нет
- Benzophenone/THF StillДокумент3 страницыBenzophenone/THF Stillbloggsjoe1970Оценок пока нет
- Stereoisomerism and Optical Isomerism Concepts ExplainedДокумент3 страницыStereoisomerism and Optical Isomerism Concepts ExplainedSanjay Mani Tripathi50% (2)
- Generator Protection Application GuideДокумент106 страницGenerator Protection Application GuideJorge Alberto Chavarría Sacasa100% (1)
- Unit2.SP - Mill.setting and ImbibitionДокумент15 страницUnit2.SP - Mill.setting and ImbibitionHari kantОценок пока нет
- Stereoisomerism VKP SirДокумент49 страницStereoisomerism VKP SirSandeep ReddyОценок пока нет
- Basara Vidyakshetram, Madhapur: Na/dry - Et OДокумент8 страницBasara Vidyakshetram, Madhapur: Na/dry - Et OvardeshОценок пока нет
- IIT-JAM-2014 Chemistry QuestionsДокумент8 страницIIT-JAM-2014 Chemistry QuestionsMahendra GanuboyinaОценок пока нет
- (02-12-14) AlkenesДокумент4 страницы(02-12-14) Alkenessasi.curieОценок пока нет
- Jee TSC Iupac Nomenclature Etoos Sheet PDFДокумент16 страницJee TSC Iupac Nomenclature Etoos Sheet PDFShadow100% (1)
- Reaction Mechanism PDFДокумент14 страницReaction Mechanism PDFSreeragОценок пока нет
- Goc FinalsheetДокумент49 страницGoc FinalsheetKartik KambleОценок пока нет
- Jitendra Hirwani: Daily Practice Problem OF Physical Chemistry For NeetДокумент8 страницJitendra Hirwani: Daily Practice Problem OF Physical Chemistry For NeetabhishekОценок пока нет
- Iupac 1Документ37 страницIupac 1shodhan shettyОценок пока нет
- ISI R: Organic ChemistryДокумент28 страницISI R: Organic Chemistrysarvesh goyalОценок пока нет
- Exam Guide: 30 MCQs, 10 MSQs, 20 NATsДокумент15 страницExam Guide: 30 MCQs, 10 MSQs, 20 NATsAnil Kumar100% (1)
- Solubility Product ProblemsДокумент4 страницыSolubility Product ProblemsT sidharth100% (1)
- NEET Periodic Table DPPДокумент9 страницNEET Periodic Table DPPArjun GudipalliОценок пока нет
- Jitendra Hirwani: Daily Practice Problem OF Physical Chemistry For NeetДокумент5 страницJitendra Hirwani: Daily Practice Problem OF Physical Chemistry For NeetabhishekОценок пока нет
- Alcohol Phenol & Ether Class-12 Jee Package PDFДокумент111 страницAlcohol Phenol & Ether Class-12 Jee Package PDFPrathmesh DixitОценок пока нет
- DPPs BOOKLET-1 CHEMISTRYДокумент21 страницаDPPs BOOKLET-1 CHEMISTRYAkkaldevi Saivinayak CRОценок пока нет
- DPP 02 Periodic Table JH Sir-3579Документ8 страницDPP 02 Periodic Table JH Sir-3579AmitSharmaОценок пока нет
- General Organic Chemistry - Sheet - 10 - 11 & 12) (Hyperconjugation & Aromaticity) Level - 1 1Документ10 страницGeneral Organic Chemistry - Sheet - 10 - 11 & 12) (Hyperconjugation & Aromaticity) Level - 1 1IznnxxkozsksnndОценок пока нет
- (Career Endeavour) IIT JAM CHEMISTRY PDFДокумент88 страниц(Career Endeavour) IIT JAM CHEMISTRY PDFKiran Mandal100% (1)
- Isomerism Page # 3Документ52 страницыIsomerism Page # 3RameshKumarОценок пока нет
- Allen DPP1 Hydrocarbons PDFДокумент6 страницAllen DPP1 Hydrocarbons PDFJannaki PvОценок пока нет
- IIT-JEE ChEmistry by N.J. sir: ORGANIC ChemIstRyДокумент32 страницыIIT-JEE ChEmistry by N.J. sir: ORGANIC ChemIstRysachin pantОценок пока нет
- Goc & Eas Test-IiДокумент7 страницGoc & Eas Test-IiAniket GuptaОценок пока нет
- GOC ExerciseДокумент45 страницGOC ExerciseAMAR DEEP SHUKLA100% (3)
- CAREER POINT PRE-MEDICAL ISOMERISM MCQДокумент20 страницCAREER POINT PRE-MEDICAL ISOMERISM MCQSanjay Mani TripathiОценок пока нет
- Goc Question Bank: Complete Course On Organic Chemistry For JEE 2020Документ8 страницGoc Question Bank: Complete Course On Organic Chemistry For JEE 2020Vishvas Ranjan SrivastavaОценок пока нет
- MKA SIR REACTION MECHANISM EXERCISE NOTESДокумент39 страницMKA SIR REACTION MECHANISM EXERCISE NOTESMrigank GuptaОценок пока нет
- Single Correct: Class: Adv - CC Time: 45 Min Class Test-3: OzonolysisДокумент4 страницыSingle Correct: Class: Adv - CC Time: 45 Min Class Test-3: Ozonolysisbruh pogОценок пока нет
- Jitendra Hirwani: Previous Year Problem Solving Iit Jee Main + Advanced Physical ChemistryДокумент14 страницJitendra Hirwani: Previous Year Problem Solving Iit Jee Main + Advanced Physical ChemistrySaptarshi DashОценок пока нет
- DPP-Alkyl and Aryl Halides - CombinedДокумент114 страницDPP-Alkyl and Aryl Halides - CombinedAffan FarukiОценок пока нет
- MCQs on AromaticityДокумент10 страницMCQs on AromaticityGaurav YadavОценок пока нет
- Solved Example: Chemistry For Neet & AiimsДокумент24 страницыSolved Example: Chemistry For Neet & AiimsAnup KОценок пока нет
- Chemical Bonding Part-01Документ40 страницChemical Bonding Part-01Mahendra ShahОценок пока нет
- Practice TestДокумент14 страницPractice TestHimanshu JindalОценок пока нет
- IIT JEE PHYSICAL & INORGANIC CHEMISTRY REVISIONДокумент5 страницIIT JEE PHYSICAL & INORGANIC CHEMISTRY REVISIONshiva royОценок пока нет
- CPP 20220411175711706233Документ139 страницCPP 20220411175711706233Ronit NigamОценок пока нет
- Jitendra Hirwani: Daily Practice Problem OF Physical Chemistry For NeetДокумент12 страницJitendra Hirwani: Daily Practice Problem OF Physical Chemistry For NeetKanthala Sai Sandesh ReddyОценок пока нет
- Topics From Botany: Kvpy Analysis: Sa StreamДокумент12 страницTopics From Botany: Kvpy Analysis: Sa StreamAnonymous EyhxoejОценок пока нет
- Worksheet On KVPY: Fiitjee BengaluruДокумент3 страницыWorksheet On KVPY: Fiitjee BengaluruRAGHUL MОценок пока нет
- Online Couseling ProcedureДокумент3 страницыOnline Couseling ProcedureRAGHUL MОценок пока нет
- Block A Collides With Block B. After The Collisions The Two Blocks Stick Together. Which of The Following Is True?Документ2 страницыBlock A Collides With Block B. After The Collisions The Two Blocks Stick Together. Which of The Following Is True?JoØrsh Ênrique Tu Xikytø NînîØflowОценок пока нет
- TNJFU UG Admission Prospectus 2018 19 TN CandidatesДокумент39 страницTNJFU UG Admission Prospectus 2018 19 TN CandidatesRAGHUL MОценок пока нет
- Disease Test ReviewДокумент2 страницыDisease Test ReviewRAGHUL MОценок пока нет
- Dictionary of QuranДокумент22 страницыDictionary of QuranSyed HarrisОценок пока нет
- Twenty Frequently Asked Questions About Mit OpencoursewareДокумент5 страницTwenty Frequently Asked Questions About Mit OpencoursewareRAGHUL MОценок пока нет
- Vci Neet PN 2019Документ1 страницаVci Neet PN 2019RAGHUL MОценок пока нет
- Fee Notification 1583Документ1 страницаFee Notification 1583RAGHUL MОценок пока нет
- 3 Trigonometric FunctionsДокумент11 страниц3 Trigonometric FunctionsSatyam PandeyОценок пока нет
- U Greg AlliedДокумент4 страницыU Greg AlliedgdmanОценок пока нет
- Central University of Tamil Nadu, Thiruvarur PROVISIONAL SELECTION LIST - Integrated M.Sc. (Chemistry)Документ1 страницаCentral University of Tamil Nadu, Thiruvarur PROVISIONAL SELECTION LIST - Integrated M.Sc. (Chemistry)RAGHUL MОценок пока нет
- MBA in Fisheries Management at TNJFU Business SchoolДокумент13 страницMBA in Fisheries Management at TNJFU Business SchoolRAGHUL MОценок пока нет
- Calculus, Differential Equations and Physics Course OverviewДокумент73 страницыCalculus, Differential Equations and Physics Course OverviewRAGHUL MОценок пока нет
- Tamil Nadu Dr. J. Jayalalithaa Fisheries University: 1 Counselling For Ug Admission 2019-2020Документ1 страницаTamil Nadu Dr. J. Jayalalithaa Fisheries University: 1 Counselling For Ug Admission 2019-2020RAGHUL MОценок пока нет
- NAHEP Invites Concept Notes for 3rd Round FundingДокумент12 страницNAHEP Invites Concept Notes for 3rd Round FundingRAGHUL MОценок пока нет
- Tamilnadu Veterinary and Animal Sciences University Undergraduate Admission 2019 - 2020Документ1 страницаTamilnadu Veterinary and Animal Sciences University Undergraduate Admission 2019 - 2020RAGHUL MОценок пока нет
- Solutions To BookДокумент48 страницSolutions To BookRAGHUL MОценок пока нет
- Advertisement English HindiДокумент2 страницыAdvertisement English HindiRAGHUL MОценок пока нет
- TNJFU UG Admission 2019Документ44 страницыTNJFU UG Admission 2019RAGHUL MОценок пока нет
- Prospectus Diploma 2019 20Документ7 страницProspectus Diploma 2019 20RAGHUL MОценок пока нет
- Vacant / Left Over Seat Matrix After Mop Up / Final Round (AIEEA UG 2019)Документ9 страницVacant / Left Over Seat Matrix After Mop Up / Final Round (AIEEA UG 2019)RAGHUL MОценок пока нет
- Quantitative Techniques in Textile EngineeringДокумент26 страницQuantitative Techniques in Textile EngineeringRAGHUL MОценок пока нет
- TNJFU UG Admission 2019Документ44 страницыTNJFU UG Admission 2019RAGHUL MОценок пока нет
- Wilo Fire Fighting BrochureДокумент20 страницWilo Fire Fighting BrochureAkhmad Darmaji DjamhuriОценок пока нет
- GR/KWH, KG/HR or Tons/Month.: ScopeДокумент5 страницGR/KWH, KG/HR or Tons/Month.: ScopeThaigroup CementОценок пока нет
- KN Yb 1000Документ13 страницKN Yb 1000taharОценок пока нет
- MDR PDFДокумент34 страницыMDR PDFJohnОценок пока нет
- 5367227Документ2 страницы5367227aliha100% (1)
- Vesda Arrange Fire Alarm SystemДокумент1 страницаVesda Arrange Fire Alarm SystemGaurav Kumar SharmaОценок пока нет
- Performance comparison of bored and excavated pilesДокумент10 страницPerformance comparison of bored and excavated pilesDavid Aponte RojasОценок пока нет
- CS2204 Analog & Digital Communication Question BankДокумент16 страницCS2204 Analog & Digital Communication Question BankJesse VincentОценок пока нет
- Thermal Barrier Coatings Seminar ReportДокумент6 страницThermal Barrier Coatings Seminar ReportGanesh NandgaonkarОценок пока нет
- Poynting OMNI A0098 BrochureДокумент2 страницыPoynting OMNI A0098 BrochurekaminareОценок пока нет
- A2 Biopharm MetalДокумент28 страницA2 Biopharm MetalThanh Nghị BùiОценок пока нет
- Think Aloud Strategy Effective for Teaching Narrative Text ComprehensionДокумент8 страницThink Aloud Strategy Effective for Teaching Narrative Text ComprehensionOxtapianus TawarikОценок пока нет
- Microelectronic Circuit Design 5th Edition Jaeger Blalock Solution ManualДокумент21 страницаMicroelectronic Circuit Design 5th Edition Jaeger Blalock Solution Manualruth100% (23)
- M6L32Документ6 страницM6L32abimanaОценок пока нет
- Fundamentals of Heat and Mass Transfer 7th Edition - Bergman, Lavine, Incropera, DeWitt (1) - p0015Документ1 страницаFundamentals of Heat and Mass Transfer 7th Edition - Bergman, Lavine, Incropera, DeWitt (1) - p0015CladyОценок пока нет
- MBA (Travel & Tourism) 1st Year Sylabus 2020-21 - 28th SeptДокумент34 страницыMBA (Travel & Tourism) 1st Year Sylabus 2020-21 - 28th SeptHimanshuОценок пока нет
- Technology: ControlsДокумент32 страницыTechnology: ControlsAli Hossain AdnanОценок пока нет
- Mech Vi Non Traditional Machining (10me665) NotesДокумент45 страницMech Vi Non Traditional Machining (10me665) Notesnikhil0% (1)
- Faraday Rotation + Verdet Constant PosterДокумент1 страницаFaraday Rotation + Verdet Constant PosterAndrew PalmerОценок пока нет
- Lee Et Al-1998-AIChE JournalДокумент10 страницLee Et Al-1998-AIChE JournalNoUrElhOdaОценок пока нет
- Structural Soils Engineer's Quick Reference GuideДокумент64 страницыStructural Soils Engineer's Quick Reference GuideGreg McNamaraОценок пока нет
- ST RDДокумент2 страницыST RDBalteshwar SinghОценок пока нет
- Sabp G 007Документ8 страницSabp G 007Li PengОценок пока нет
- Dynamic Programming Algorithm Explained in ECE 551 LectureДокумент11 страницDynamic Programming Algorithm Explained in ECE 551 Lectureadambose1990Оценок пока нет
- Tecquipment - Flumes - Data SheetДокумент3 страницыTecquipment - Flumes - Data SheetArthur BritoОценок пока нет