Академический Документы
Профессиональный Документы
Культура Документы
Kidney Diseases Associated With Human Immunodeficiency Virus Infection
Загружено:
VISHWANATH MARSHIVANIKARОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Kidney Diseases Associated With Human Immunodeficiency Virus Infection
Загружено:
VISHWANATH MARSHIVANIKARАвторское право:
Доступные форматы
The n e w e ng l a n d j o u r na l of m e dic i n e
Review Article
Julie R. Ingelfinger, M.D., Editor
Kidney Diseases Associated with Human
Immunodeficiency Virus Infection
Scott D. Cohen, M.D., M.P.H., Jeffrey B. Kopp, M.D., and Paul L. Kimmel, M.D.
H
ighly active antiretroviral therapy has led to dramatic im- From the Division of Renal Diseases and
provement in the life expectancy of persons with human immunodefi- Hypertension, Department of Medicine,
George Washington University, Washing-
ciency virus (HIV) infection.1-3 Approximately 36.7 million people live with ton, DC (S.D.C., P.L.K.); and the National
HIV infection worldwide,1,2,4,5 and there were approximately 2.1 million cases of in- Institute of Diabetes and Digestive and
cident HIV infection globally in 2015.5 Almost three quarters of HIV-infected persons Kidney Diseases, National Institutes of
Health, Bethesda, MD (J.B.K., P.L.K.). Ad-
live in sub-Saharan Africa.1,2,4,6 Although 18.2 million people worldwide were receiv- dress reprint requests to Dr. Kimmel at
ing antiretroviral therapy by 2016,5 only 40% of HIV-infected persons in sub-Saharan National Institute of Diabetes and Diges-
Africa received antiretroviral therapy as of 2014.7 The prevalence of HIV infection is tive and Kidney Diseases, National Insti-
tutes of Health, Rm. 6707, Democracy Blvd.,
much lower in the United States than in sub-Saharan Africa. Approximately 1.2 mil- Bethesda, MD 20892, or at kimmelp@
lion persons in the United States have HIV infection, and the annual incidence has extra.niddk.nih.gov.
been stable at approximately 50,000 infections over the past decade.5,8 N Engl J Med 2017;377:2363-74.
Kidney disease, which is a common complication of HIV infection and its treat- DOI: 10.1056/NEJMra1508467
ment, may shorten the lifespan of patients.4,9,10 Soon after the index cases of the Copyright © 2017 Massachusetts Medical Society.
acquired immunodeficiency syndrome (AIDS) were identified in 1980, various
kidney diseases associated with AIDS were recognized.10 The spectrum of HIV-
associated renal diseases includes diseases that are directly associated with infec-
tion, those that are linked to the systemic immune response to infection, those
that develop as a consequence of superinfections, and those that are associated
with the treatment of HIV infection (Table 1).10 Since the introduction of molecular
tools to detect HIV within tissues, our understanding of the pathogenesis of com-
mon kidney diseases, such as focal segmental glomerulosclerosis and immune-
complex renal disease, in persons with HIV infection has improved. Over the past
two decades, antiretroviral therapy has converted HIV infection to a chronic ill-
ness, with associated changes in the incidence, type, and severity of HIV-associated
kidney diseases. Current antiretroviral therapy regimens suppress viral replication,
but this treatment may result in chronic inflammation, premature aging, and
metabolic disorders (e.g., diabetes, hyperlipidemia, and abnormal body fat compo-
sition) — conditions that are associated with chronic kidney disease.11-15
E va luat ion of R ena l S y ndrome s in HI V Infec t ion
Patients with HIV infection are at increased risk for both acute kidney injury and
chronic kidney disease.4,9,10,12,16-18 Untreated HIV infection, as well as antiretroviral
therapy, are associated with kidney disease. Antiretroviral therapy is a double-
edged sword: although it can lead to improvement in the life expectancy of persons
with HIV infection, it can also increase clinical uncertainty regarding changes in
renal function in this population. The approaches to evaluating acute kidney injury
and chronic kidney disease are shown in Figure 1. Given the breadth of causes of
these kidney diseases, a kidney biopsy may be necessary to establish a diagnosis.
Microalbuminuria, a key sign of kidney disease but also a manifestation of the
metabolic syndrome and vascular dysfunction, is present in 10 to 15% of patients
n engl j med 377;24 nejm.org December 14, 2017 2363
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
The n e w e ng l a n d j o u r na l of m e dic i n e
Table 1. Kidney Diseases in Patients with Human
with HIV infection.19 The prevalence of a reduced
Immunodeficiency Virus Infection. glomerular filtration rate is 2 to 10% but varies
across populations.20 Depletion of intravascular
Acute kidney injury volume is probably the most common cause of
Prerenal azotemia acute kidney injury.16,17 Hospitalized patients
Acute tubular necrosis with HIV infection who have sepsis or who are
Rhabdomyolysis receiving nephrotoxic medications are at in-
Antiretroviral therapy–associated acute kidney injury creased risk for acute tubular necrosis and acute
Tubulointerstitial nephritis interstitial nephritis. Acute kidney injury and
Immune reconstitution syndrome
chronic kidney disease may be closely inter-
twined over time; chronic kidney disease often
HIV-associated nephropathy
precedes acute kidney injury but could also de-
HIV immune-complex kidney disease
velop or worsen after acute kidney injury, fre-
IgA nephropathy
quently culminating in end-stage renal disease.18,21
Postinfectious glomerulonephritis Equations for calculating the estimated glo-
Mesangial proliferative glomerulonephritis merular filtration rate have been evaluated in
Lupus-like glomerulonephritis patients with HIV infection22,23; the estimated
Membranoproliferative glomerulonephritis glomerular filtration rate can be calculated on
Cryoglobulinemic glomerulonephritis the basis of serum creatinine concentration or,
Other glomerulonephridites in patients with reduced muscle mass, serum
Thrombotic microangiopathies cystatin C level and provides clinical guidance
Urinary tract obstruction
for drug dosing. Trimethoprim, cobicistat, and
HIV integrase inhibitors inhibit proximal renal
Bladder outlet obstruction
tubular secretion of creatinine, which may in-
Ureteral obstruction
crease the serum creatinine concentration24 with-
Intrinsic: fungus balls, blood clots out changing the glomerular filtration rate. The
Extrinsic: retroperitoneal fibrosis, lymphadenopathy serum creatinine concentration does not typi-
Chronic kidney disease cally increase more than 15 to 30% with such
HIV-associated nephropathy medications.25 Greater increases in the serum
HIV immune-complex kidney disease creatinine concentration should prompt an eval-
Antiretroviral therapy–associated chronic kidney disease uation for acute kidney injury.
Tubulointerstitial nephritis Estimation of glomerular filtration rate that
Crystal nephropathy
is based on the cystatin C level may help to iden-
tify the development of chronic kidney disease,
Tenofovir disoproxil fumarate–induced nephrotoxicity
particularly in patients with moderately dimin-
Tubulointerstitial renal disease
ished creatinine-based estimated glomerular fil-
Diffuse infiltrative lymphocytosis syndrome
tration rate and muscle wasting, in whom the
Opportunistic infections of the kidney parenchyma serum creatinine concentration may not accurately
Viral infections reflect the glomerular filtration rate.9 Because
Cytomegalovirus cystatin C levels may be elevated as a result of
Parvovirus inflammation, the cystatin C–based estimated
Herpes simplex glomerular filtration rate must be used cau-
Other infections tiously.
Fungal infections
Mycobacterial infections, typical and atypical HI V-A sso ci ated Nephropath y
Mycoplasma
Before the availability of antiretroviral therapy,
Microsporidia
HIV-associated nephropathy, initially reported
Bacterial pyelonephritis by research groups in Miami and New York in
Infiltrative lesions of the kidney 1984,26-28 typically presented with nephrotic-range
Lymphoma proteinuria and decreased renal function.4,9,10
Kaposi’s sarcoma Currently, presentation varies depending on
whether antiretroviral therapy has been provided.
2364 n engl j med 377;24 nejm.org December 14, 2017
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
HIV-Associated Kidney Diseases
Acute Kidney Injury Chronic Kidney Disease
Acute tubular necrosis HIV-associated nephropathy
Prerenal
• Granular or muddy brown casts • Nephrotic-range proteinuria
• Fractional excretion of sodium, >2% • Volume depletion • High HIV viral load
• Bland urine sediment • Low CD4 count
• Fractional excretion
of sodium, <1%
Sepsis Medication Pigment
associated nephrotoxicity nephropathy Combination antiretroviral therapy
nephropathy
Thrombotic microangiopathy • Subnephrotic proteinuria
• Microangiopathic hemolytic anemia • Controlled viral load and CD4 count
• Thrombocytopenia
• Hematuria
• Proteinuria Interstitial Crystalluria • Mitochondrial
nephritis toxicity
• Fanconi’s
Acute interstitial nephritis Intrinsic syndrome
Renal
• Active urine sediment
• Pyuria Other kidney syndromes
• White-cell casts
• Diabetic kidney diseases
• Hypertensive kidney diseases
Medications Infection related • Focal segmental glomerulosclerosis
HIV-associated immune-complex
renal disease
• Active urine sediment
• Proteinuria
• Microscopic hematuria
• Red-cell casts Postrenal
• Hypocomplementemia Obstructive
• Screen for hepatitis and other
coinfections
Figure 1. Approaches to Evaluating Kidney Disease in Patients with HIV Infection.
In the evaluation of acute kidney injury in patients with human immunodeficiency virus (HIV) infection, the differential diagnosis of a
sudden reduction in the glomerular filtration rate is made with a close microscopic examination of a urine sample to help differentiate
classic prerenal, intrinsic renal, and postrenal obstructive causes. Evaluation of urinary osmolality and sodium and creatinine concentra-
tion may add value. The evaluation of chronic kidney disease in HIV-infected patients requires a careful history and physical examination
to assess traditional risk factors for renal disease, in addition to unique clinical characteristics of HIV-associated renal diseases. Evalua-
tion of urinary protein excretion is helpful in establishing diagnostic categories. A renal biopsy is often necessary to confirm the diagno-
sis and inform prognosis and treatment. Acute tubular necrosis and acute interstitial nephritis are usually manifestations of acute kidney
injury. HIV-associated nephropathy, HIV immune-complex kidney disease, kidney disease of thrombotic microangiopathy, and nephro-
toxicity can be manifestations of either acute kidney injury or chronic kidney disease.
Edema is variably present, because some patients scarring, with only some glomeruli affected.
with HIV-associated renal disease may have malnu- Other histologic features include microcystic re-
trition or salt wasting from renal tubular injury.29 nal tubular dilatation, interstitial inflammation,
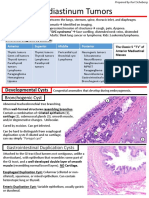
HIV-associated nephropathy affects all renal fibrosis, and tubuloreticular inclusion bodies,
tissue compartments, including glomeruli, tubules, which are detected occasionally in glomerular
and interstitium (Figs. S1 and S2 in the Supple- endothelial cells by electron microscopy.4,9,10
mentary Appendix, available with the full text of The prevalence of HIV-associated nephropa-
this article at NEJM.org). A collapsing form of thy has decreased dramatically since the intro-
focal segmental glomerulosclerosis is a typical duction of antiretroviral therapy and consequent
manifestation of HIV-associated nephropathy effective suppression of viral replication.4,31 Be-
and is characterized by an increased number of fore the antiretroviral therapy era, the prevalence
cells in the podocyte compartment; these cells of HIV-associated nephropathy was 3.5 to 10.0%
may be podocyte progenitors.30 Focal segmental among HIV-infected persons in the United
glomerulosclerosis shows segmental glomerular States.4,10,31 Over the past 20 years, the incidence
n engl j med 377;24 nejm.org December 14, 2017 2365
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
The n e w e ng l a n d j o u r na l of m e dic i n e
of end-stage renal disease attributed to HIV in- frequencies are approximately 50% among West
fection has declined and then plateaued, whereas Africans and approximately 35% among African
the prevalence of end-stage renal disease in this Americans. Approximately 13% of African Amer-
patient population continues to increase, be- icans are at increased genetic risk for chronic
cause patients live longer with effective antiret- kidney disease.4
roviral therapy.32 The annual incidence of 800 to APOL1 renal risk variants are strongly associ-
900 cases of HIV-associated end-stage renal ated with HIV-associated nephropathy.37,38 Inter-
disease has been stable for several years.32 actions between gene and environment may
The prevalence of HIV-associated nephropa- modify the strength of the associations. South
thy varies worldwide, with the highest rates re- African patients with HIV-associated nephropa-
ported in sub-Saharan Africa.6 In the United thy who have a single APOL1 risk allele have a
States, HIV-associated nephropathy occurs pri- higher risk of kidney disease than patients who
marily in persons of African descent, particu- have no APOL1 risk alleles, which is consistent
larly in those with markedly reduced CD4 counts with a dominant gain-of-function effect of the
and elevated viral loads.4 HIV-associated nephrop- APOL1 risk allele, as seen in animal models,38-41
athy probably arises because of complex interac- rather than a recessive loss-of-function effect.
tions among host factors (especially genetics), HIV infection stimulates interferon expres-
pathogen characteristics (particularly renal viral sion.42 Expression of APOL1, an innate immunity
protein expression), and environmental and be- gene, is stimulated by interferon,43 which prob-
havioral factors (the most important of which ably explains in part why associations with
may be access to care and antiretroviral therapy APOL1 renal risk variants are so strong in HIV
with effective viral suppression) (Fig. 2). infection. APOL1 risk alleles are incompletely
In the kidneys, HIV RNA has been shown to penetrant, as evidenced by the fact that kidney
be localized to podocytes and tubular epithelial disease does not develop in most carriers of two
cells, which perhaps explains why these cells risk alleles, which suggests that modulation by
show striking abnormalities in HIV-associated environmental or other genetic factors is also
nephropathy. The HIV regulatory protein Nef needed (Fig. 2). However, in some patients, in-
and the HIV accessory protein Vpr, when overex- creased expression of risk alleles and their prod-
pressed in mice, reproduce the HIV-associated ucts, which is stimulated by interferon, may be
nephropathy syndrome, which suggests that these sufficient to induce glomerulopathy.29 Recent
proteins play pathogenic roles in HIV-associated evidence suggests that serum urokinase-type
nephropathy.4,10 plasminogen activator receptor, an inflamma-
The genetic predisposition to HIV-associated tion marker, may interact with APOL1, thereby
nephropathy among patients of African descent activating the aVb3 integrin receptor, which is
is due largely to variants in the APOL1 gene, en- expressed in podocytes, and perhaps promoting
coding apolipoprotein L1.33,34 APOL1 is a minor kidney disease in genetically susceptible per-
protein constituent of particular high-density sons.44 In APOL1 transgenic mice, reduction in
lipoprotein particles that comprise two forms of APOL1 gene expression is associated with im-
trypanosome lytic factor.35 In vitro data show proved renal histologic findings and less azote-
that APOL1 variants help in killing Trypanosoma mia and albuminuria.41 Because the nephropa-
brucei, the cause of African sleeping sickness.33,36 thies that are most strongly associated with
Recent findings regarding the APOL1 renal risk APOL1 variants are podocytopathies,45 it is likely
variants G1 and G2 (in contrast to the common that the expression and products of APOL1 vari-
allele G0) implicate both variants in the resis- ants damage podocytes and possibly other renal
tance to subspecies of T. brucei, which suggests cells. Emerging evidence suggests a direct role
that the variants evolved to kill T. brucei subspe- of the products of APOL1 variants in nephro-
cies.36 Both variants are limited to populations pathogenesis,40,41 including alterations in mem-
of sub-Saharan descent.4 brane ion flux46; altered mitochondrial,47 en-
APOL1 risk variants are also associated with dolysosomal, and autophagic function39,41; and
non-HIV chronic kidney disease, including focal increased cellular inflammatory pathways, in-
segmental glomerulosclerosis and hypertension- cluding activation of protein kinase R.29,41 APOL1
associated arterionephrosclerosis.33,37 Risk-allele variants have not been associated with an in-
2366 n engl j med 377;24 nejm.org December 14, 2017
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
HIV-Associated Kidney Diseases
Host
factors
• Genetics – APOL1
variants
• Other possible
Pathogen factors
characteristics
Environmental and
behavioral factors
• Nephrotoxins
• Coinfections
• Nutrition
Access and
adherence to
combination
antiretroviral
therapy
Increased Decreased
likelihood of HIV-associated likelihood of HIV-associated
nephropathy nephropathy
Figure 2. Pathogenesis of HIV-Associated Nephropathy.
The pathogenesis of HIV-associated nephropathy involves the complex interaction of host and environmental fac-
tors, pathogen characteristics, and patient access to antiretroviral therapy. In most cases, kidney disease does not
develop in persons who have two APOL1 risk alleles, which suggests that other factors are necessary to transform
genetic risk into expression of kidney disease, such as environmental factors, coinfections, coexisting medical ill-
nesses, or treatment effects. HIV-associated nephropathy is unique in that treatment plays a key role in mediating
expression of nephropathy. Increased APOL1 gene expression, stimulated by interferon, may be sufficient to induce
glomerulopathy. Additional factors may synergize with APOL1 variants, in which case APOL1 variants could be seen
as susceptibility factors. APOL1 variants probably damage podocytes and other renal cells. Although injury mecha-
nisms are not well understood, evidence points to alterations in ion flux across the plasma membrane; altered endoly-
sosomal, mitochondrial, and autophagic function; and increased cellular inflammatory pathways, including activation
of protein kinase R and effects of interferon as key pathogenic factors.
creased risk of HIV-associated immune-complex retroviral therapy, HIV-associated nephropathy
renal disease.48,49 typically progressed rapidly to end-stage renal
disease10; subsequently, HIV-associated nephrop-
athy was considered to be an indication to initi-
T r e atmen t of HI V-A sso ci ated
Nephropath y ate antiretroviral therapy.51 This approach has
been superseded by a preventive approach in
The use of highly active antiretroviral therapy is which antiretroviral treatment is recommended
central to the effective treatment of HIV-associ- for all HIV-infected persons; such treatment may
ated nephropathy,4 especially because the kid- prevent HIV-associated nephropathy from pro-
neys are a reservoir for HIV nucleic acids, even gressing.52 Studies in sub-Saharan African popu-
during therapy50; however, the importance of lations showed increased glomerular filtration
such agents in reducing kidney disease has not rates in affected patients after 2 to 5 years of
been shown in well-controlled, randomized, antiretroviral therapy.53,54 Patients with reduced
clinical trials. Before the introduction of anti- glomerular filtration rates may require dose ad-
n engl j med 377;24 nejm.org December 14, 2017 2367
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
The n e w e ng l a n d j o u r na l of m e dic i n e
justments of antiretroviral medications, especially tion of circulating immune complexes that are
nucleoside reverse transcriptase inhibitors.55 deposited in the glomerular capillary tuft, and
Glucocorticoids may play a role in the treat- in situ deposition of antibodies that bind to glo-
ment of HIV-associated nephropathy, but sup- merular antigens and activate complement.49,61,62
porting evidence is limited.10,56 Patients with Types of HIV immune-mediated kidney dis-
histologic evidence of dense tubulointerstitial ease include diverse renal histologic manifes
inflammatory infiltrates may be more likely to tations — HIV-associated IgA nephropathy,
benefit. Physicians should carefully consider the postinfectious glomerulonephritis, lupus-like glo-
risks and benefits when prescribing immunosup- merulonephritis, membranoproliferative glomer-
pressive therapy to immunocompromised hosts. ulonephritis, cryoglobulinemic glomerulonephri-
Renin–angiotensin pathway antagonists may tis, mesangial proliferative glomerulonephritis,
help decrease proteinuria and slow progressive membranous nephropathy, and other conditions
fibrosis.4,10,51 Such therapy in transgenic mouse that are usually characterized by glomerular
models of HIV-associated nephropathy slowed hypercellularity, inflammation, and varying de-
the progression of glomerular and tubular dis- grees of fibrosis and scarring (Table 1).49,59,61-63
ease.57,58 Evidence-based blood-pressure goals Clinical features include proteinuria (often in
for HIV-associated nephropathy — which are the subnephrotic range), reduced glomerular
important to set when antagonists of the renin– filtration rate (usually with a progressive course),
angiotensin pathway are used — have not been hypocomplementemia, and abnormal urinary
established, but the goals may mirror those for sediment (typically with hematuria and red-cell
other proteinuric kidney diseases. casts).49 Although it is difficult in clinical set-
tings to determine whether a glomerular disease
is intrinsically linked to the viral infection, clini-
HI V-A ssoci ated Immune-Medi ated
K idne y Dise a se cal and experimental evidence exists to confirm
the relationship of specific HIV-associated circu-
Since the beginning of the HIV pandemic, pro- lating immunoreactants, including idiotypic anti-
liferative glomerulonephritides have constituted bodies and antibodies directed against gp120,
a substantial proportion of cases of HIV-associ- gp41, and p24, with the development of glomeru-
ated kidney disease.10,27,28,49,59-63 Studies of renal- lonephritis.49,61,62 Coinfection with other patho-
biopsy specimens from U.S. patients with HIV gens, such as hepatitis C virus, may influence
infection have shown that the proportion of the pathogenesis of HIV immune-mediated kid-
glomerular diseases other than HIV-associated ney disease.49 Immune-mediated mechanisms
nephropathy has increased over the past two may also underlie inflammatory cellular infiltra-
decades.49,60 Although it is likely that selection tion, causing tubulointerstitial renal diseases
bias is a factor in renal-biopsy registries, HIV such as diffuse infiltrative lymphocytosis.64
immune-mediated kidney disease has become Renal biopsy is necessary to establish a di-
the most common histopathological diagnosis agnosis of HIV immune-mediated kidney dis-
in several studies of biopsy specimens from HIV- ease.49,65 Determining precise etiologic relation-
infected patients.49 The reasons underlying the ships between the virus, immune system, and
increasing relative prevalence of HIV immune- kidney injury in a particular patient requires re-
mediated kidney disease are unknown but may search methods, such as immunoanalyses of
reflect modulation of the immune system in- tissue proteins, that are not usually possible in
duced by antiretroviral therapy, which can lead a clinical setting.49 The most effective treatment
to immune reconstitution and immune-complex of HIV immune-mediated kidney disease in the
deposition, or it could be due to the decline in absence of evidence from randomized, controlled
the incidence of HIV-associated nephropathy.49 trials is unknown but should include antiretro-
According to current thinking, the pathogenesis viral therapy. The role of immunosuppression in
of HIV immune-mediated kidney disease involves the treatment of HIV immune-mediated kidney
the following distinct mechanisms: specific anti- disease is controversial. In patients with cres-
body responses to HIV epitopes and polyclonal centic glomerulonephritis, immunosuppression
gammopathy, both of which result in the forma- with glucocorticoids with or without additional
2368 n engl j med 377;24 nejm.org December 14, 2017
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
HIV-Associated Kidney Diseases
immunomodulatory therapy may be appropriate chronic medical conditions associated with renal
if there is no contraindication.49 disease in HIV-infected patients as well as in the
general population.11-14,51 Studies of renal-biopsy
specimens from HIV-infected patients suggest
Thrombo t ic Microa ngiopath y
in HI V-A sso ci ated R ena l Dise a se that the prevalences of diabetic nephropathy,
hypertension-associated arterionephrosclerosis,60
Thrombotic microangiopathy is another cause of and noncollapsing focal segmental glomerulo-
renal disease associated with HIV infection66,67; sclerosis60,71 are increasing because these patients
this condition manifests as thrombocytopenia are living longer and these common proteinuric
and microangiopathic hemolytic anemia, with or chronic kidney diseases have the opportunity
without neurologic deficits and fever. Examina- to develop.60 Accelerated aging associated with
tion of peripheral-blood smears to detect schisto- chronic HIV infection may drive glomerular and
cytes and reduced serum haptoglobin level help vascular changes that are synergistic with diabe-
in determining the diagnosis. Urinalysis often tes, hypertension, and APOL1 variants (Fig. 2);
reveals hematuria and proteinuria. HIV has indi- however, further studies are required to estab-
rect cytopathic effects that precipitate endothe- lish causal relationships. The effect of improved
lial injury, leading to thrombotic microangiop- glycemic and blood-pressure control on renal
athy. HIV p24 antigen has been identified in outcomes in this patient population is unknown.
endothelial cells of patients with thrombotic
microangiopathy.68 Coexisting medical condi- Drug -Induced T ubul a r
tions, including opportunistic infections and a nd In ter s t i t i a l R ena l Inj ur y
malignant conditions, or antiretroviral therapy
may also be involved in the pathogenesis of Renal disease may develop or worsen during anti-
thrombotic microangiopathy. retroviral therapy, which often presents diag-
The development of thrombotic microangiopa- nostic challenges. Antiretroviral therapy can be
thy is associated with high plasma HIV viral associated with nephrotoxic effects, including
loads and decreased CD4 counts.68,69 The preva- acute and chronic renal disease.69,72 The protease
lence of thrombotic microangiopathy in the inhibitors indinavir and atazanavir are insoluble
United States has decreased over the past two in alkaline urine and promote crystalluria and
decades, with the decrease probably attributable occasionally crystal nephropathy.72 The use of
to effects of antiretroviral therapy.66,67 Therapeu- medication algorithms73,74 may reduce nephro-
tic options for HIV-associated thrombotic micro- toxic effects in populations of HIV-infected pa-
angiopathy include treatment of the underlying tients. The diagnosis of crystal nephropathy and
viral infection and plasma exchange. Eculizumab granulomatous interstitial nephritis is confirmed
has been successfully used to treat thrombotic by renal biopsy (Fig. S3 in the Supplementary
microangiopathy that is resistant to plasma ex- Appendix).65,72,75 Treatment of crystal nephropa-
change.70 In the absence of data from random- thy involves changing the patient’s antiretroviral
ized, controlled trials, immunosuppression for regimen, since alternative antiretroviral regimens
refractory thrombotic microangiopathy should are almost always available. Glucocorticoids have
be considered only after the potential risks and been used in uncontrolled studies to treat the
benefits in immunocompromised patients are associated interstitial nephritis, but results have
carefully weighed. been inconsistent.
Tenofovir disoproxil fumarate, which is cleared
by the kidneys through glomerular filtration and
O ther Chronic K idne y Dise a se s
in Pat ien t s w i th HI V Infec t ion active proximal renal tubular secretion, is a first-
line HIV therapy. Tenofovir disoproxil fumarate
Prolonged therapy with antiretroviral drugs has enters tubular cells from the pericellular space
been associated with both increased life expec- through organic anion transporters 1 and 3 and is
tancy 2,3,5 and the development of the metabolic secreted into the tubular lumen through multi-
syndrome, diabetes mellitus, and hypertension.69 drug resistance proteins 2 and 4 and the breast
As a result, there is an increased prevalence of cancer resistance protein 4 (Fig. 3).76 Tenofovir
n engl j med 377;24 nejm.org December 14, 2017 2369
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
The n e w e ng l a n d j o u r na l of m e dic i n e
Basolateral Luminal
B LO O D P R OX I M AL T U B U L E C E L L TUBULAR
F L U ID
Tenofovir
disoproxil Accumulation of
fumarate tenofovir disoproxil
fumarate
Human organic Mitochondrial
anion transporter 1 toxicity Multidrug resistant
protein 4
Human organic
anion transporter 3
Multidrug resistant
protein 2
Tenofovir
alafenamide
Figure 3. Handling of Tenofovir by Proximal Tubular Cells.
Tenofovir disoproxil fumarate is taken up by the organic anion transporters 1 and 3 on the basolateral membrane of the proximal tubu-
lar cell and secreted into the tubular lumen through the multidrug resistance proteins 2 and 4. The newer agent, tenofovir alafenamide,
is not a substrate for organic anion transporters 1 and 3 and thus has less cellular uptake, which probably accounts for its having less
nephrotoxicity than tenofovir disoproxil fumarate, as reported previously.76,77 Intracellular accumulation of tenofovir can cause mitochon-
drial toxic effects and proximal tubular injury, which can lead to Fanconi’s syndrome. Clinical characteristics include proteinuria, normo-
glycemic glucosuria, hypophosphatemia and phosphaturia, aminoaciduria, and uricosuria and hypouricemia. Nephrogenic diabetes in-
sipidus and acute tubular necrosis have also been described.72,76
disoproxil fumarate can cause renal tubular dys- ated with fewer nephrotoxic effects. Tenofovir
function, which leads to Fanconi’s syndrome, alafenamide is not a substrate for organic anion
nephrogenic diabetes insipidus, acute tubular transporters 1 and 3 and thus does not accumu-
necrosis, and ultimately chronic kidney disease late in proximal tubular cells to the extent that
(Figs. S4 and S5 in the Supplementary Appendix).4 tenofovir disoproxil fumarate does.77 Tenofovir
Fanconi’s syndrome induced by tenofovir diso- alafenamide has more potent anti–HIV-1 activity
proxil fumarate results from the intracellular and increased intracellular accumulation than
accumulation of the drug’s metabolites that oc- tenofovir disoproxil fumarate, which yields lower
curs because of the relatively efficient uptake of levels of tenofovir in plasma.76
tenofovir disoproxil fumarate into the proximal The development of the immune reconstitu-
tubular cell through organic anion transporters tion syndrome after initiation of antiretroviral
and the less efficient efflux from the proximal therapy may be associated with acute kidney
tubular cell through multidrug resistance pro- injury.78 Renal pathologic features of the im-
teins. Its proximal tubular cell efflux is also in- mune reconstitution syndrome include dense
hibited by commonly used antiretroviral medica- interstitial infiltrates that result from immune
tions, including ritonavir, thus increasing the system reactivation. The immune reconstitution
risk of toxic effects. syndrome and the diffuse infiltrative lymphocy-
The prodrug tenofovir alafenamide is associ- tosis syndrome64 should be considered in the dif-
2370 n engl j med 377;24 nejm.org December 14, 2017
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
HIV-Associated Kidney Diseases
ferential diagnosis of tubulointerstitial nephritis fumarate and ritonavir-boosted protease inhibi-
in HIV-infected patients who are receiving anti- tors, were associated with an increased risk of
retroviral therapy (Table 1). disease progression.
Measurement of urinary biomarkers has been
reported to be useful in the early detection of Di a lysis a nd HI V Infec t ion
nephrotoxic effects.79-81 Patients with longer cumu-
lative exposure to tenofovir disoproxil fumarate The life expectancy of patients with HIV-associ-
were shown to have progressively higher urinary ated end-stage renal disease has increased mark-
α1-microglobulin levels, whereas patients whose edly over the past 20 years.32 Although the inci-
treatment was discontinued had progressively dence of HIV-associated end-stage renal disease
lower levels — a finding that highlights the has plateaued, the prevalence of HIV-infected
promise of urinary proteomics in the detection patients undergoing dialysis in the United States
of early renal tubular injury.81 continues to increase.32 Survival among patients
with HIV infection who undergo dialysis is simi-
lar to that among patients without HIV infec-
Di agnosis a nd M a nagemen t
of Chronic K idne y Dise a se tion who undergo dialysis.32 Survival among HIV-
infected patients does not differ between those
Determining the cause of incipient and progres- who undergo peritoneal dialysis and those who
sive renal disease in patients with HIV infection undergo hemodialysis.85 Standard universal pre-
who are receiving antiretroviral therapy may be cautions apply to HIV-infected patients undergo-
difficult. Clues can be found in the amount and ing dialysis, and these patients do not require
character of urine protein excretion and in the isolation. Peritoneal dialysate from HIV-infected
urine sediment (Fig. 1). A renal biopsy may be persons should be considered to be a contami-
necessary to determine diagnosis, prognosis, nated body fluid, because HIV can survive in the
and management.65 effluent and tubing for variable periods.86
Screening for chronic kidney disease is rec-
ommended in persons with newly diagnosed K idne y T r a nspl a n tat ion
HIV infection51 and includes urinalysis and mea- a nd HI V Infec t ion
surement of serum creatinine concentration and
urine protein and albumin levels in random A dramatic advance in the care of patients with
urine samples. When the ratio of protein to HIV-associated end-stage renal disease occurred
creatinine is elevated in the urine, higher urine after kidney transplantation was shown to be via-
albumin-to-protein ratios help to distinguish ble.50,87,88 Although the rates of acute rejection are
glomerular from tubular proteinuria. Urine dip- higher among HIV-infected transplant recipients
stick testing was shown to be less sensitive than than among other kidney transplant recipients,
quantitative urine protein measurement for iden- outcomes with respect to patient and allograft
tifying renal disease in HIV-infected patients.82 survival are similar.87,88 Candidates for renal
Taken together, the estimated glomerular filtra- transplantation who have HIV infection should
tion rate and the urine albumin-to-creatinine have CD4 counts higher than 200 cells per cubic
ratio provide a means to determine the stage of millimeter and undetectable viral loads.87 Inter-
chronic kidney disease and may yield important actions between antiretroviral drugs and the im-
prognostic and therapeutic guidance.83 munosuppressive drugs needed to prevent allo
The effect of antiretroviral therapy on the graft rejection necessitate close monitoring of the
progression of chronic kidney disease was evalu- immunosuppressive drug level.
ated in an observational cohort.84 Antiretroviral Findings from a recent study suggest that kid-
therapy was associated with slower progression neys from donors with variant APOL1 alleles are
of chronic kidney disease, as assessed by the associated with poorer recipient outcomes than
estimated glomerular filtration rate. Black race, kidneys from donors without the variant alleles,
lower CD4 count, higher HIV viral loads, hepati- but the role of genetic screening needs clarifi-
tis C virus coinfection, and particular antiretro- cation.89 The HIV Organ Policy Equity (HOPE)
viral medications, including tenofovir disoproxil Act allows the transplantation of kidneys and
n engl j med 377;24 nejm.org December 14, 2017 2371
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
The n e w e ng l a n d j o u r na l of m e dic i n e
other organs from HIV-infected donors to HIV- of HIV-infected patients with immune-complex
positive recipients under the auspices of a re- renal disease, which may be more prevalent as a
search program,90 thereby potentially increas- result of generally successful HIV therapy. Kid-
ing the donor pool.91 ney transplantation holds promise to increase
the quantity and quality of life for patients with
HIV infection in whom end-stage renal disease
C onclusions
develops. Additional research, including well-
The spectrum of HIV-associated kidney disease designed randomized, controlled trials, is need-
has changed over the past three decades owing ed to determine effective therapeutic approaches
to far better therapy for HIV infection. During to treating the diverse renal manifestations of
this period, evidence of nephrotoxic effects of HIV infection and improve the outcomes of
antiretroviral therapy and the prevalence of kid- treatment.
ney diseases resulting from aberrant metabolism The opinions expressed in this article are those of the authors
and aging have increased. HIV-associated ne- and do not necessarily reflect those of the National Institute of
Diabetes and Digestive and Kidney Diseases (NIDDK), the Na-
phropathy, although much less frequent now tional Institutes of Health, the Department of Health and Human
than 20 years ago, is currently understood to Services, or the U.S. Government.
arise from complex interactions among the virus No potential conflict of interest relevant to this article was
reported.
(renal-cell infection and the effect of viral pro- Disclosure forms provided by the authors are available with
teins), host genotype, host response (interferon the full text of this article at NEJM.org.
production), and perhaps most importantly, effec- We thank Dr. Avi Rosenberg of the NIDDK, Bethesda, MD,
and Drs. Samih Nasr, Joseph Grande, Priya Alexander, and Mary
tive treatment. Fidler of the Mayo Clinic, Rochester, MN, for the provision of
There is increasing recognition of a subgroup clinical photomicrographs.
References
1. UNAIDS. UNAIDS fact sheet 2014 9. Estrella MM, Fine DM. Screening for 18. Chawla LS, Eggers PW, Star RA, Kim-
(http://files.unaids.org/en/media/unaids/ chronic kidney disease in HIV-infected mel PL. Acute kidney injury and chronic
contentassets/documents/factsheet/2014/ patients. Adv Chronic Kidney Dis 2010;17: kidney disease as interconnected syn-
20140716_FactSheet_en.pdf). 26-35. dromes. N Engl J Med 2014;371:58-66.
2. GBD 2015 HIV Collaborators. Esti- 10. Kimmel PL, Barisoni L, Kopp JB. 19. Hadigan C, Edwards E, Rosenberg A,
mates of global, regional, and national Pathogenesis and treatment of HIV-associ- et al. Microalbuminuria in HIV disease.
incidence, prevalence, and mortality of ated renal diseases: lessons from clinical Am J Nephrol 2013;37:443-51.
HIV, 1980-2015: the Global Burden of Dis- and animal studies, molecular pathologic 20. Overton ET, Nurutdinova D, Freeman J,
ease Study 2015. Lancet HIV 2016;3(8): correlations, and genetic investigations. Seyfried W, Mondy KE. Factors associated
e361-e387. Ann Intern Med 2003;139:214-26. with renal dysfunction within an urban
3. Bärnighausen T. The HIV treatment 11. Kooman JP, van der Sande FM, Leunis- HIV-infected cohort in the era of highly
cascade and antiretroviral impact in dif- sen KM. Kidney disease and aging: a recip- active antiretroviral therapy. HIV Med 2009;
ferent populations. Curr Opin HIV AIDS rocal relation. Exp Gerontol 2017;87:156-9. 10:343-50.
2015;10:391-4. 12. Nadkarni GN, Konstantinidis I, Wyatt 21. Choi AI, Li Y, Parikh C, Volberding
4. Rosenberg AZ, Naicker S, Winkler CA, CM. HIV and the aging kidney. Curr Opin PA, Shlipak MG. Long-term clinical con-
Kopp JB. HIV-associated nephropathies: HIV AIDS 2014;9:340-5. sequences of acute kidney injury in the
epidemiology, pathology, mechanisms and 13. Lake JE, Currier JS. Metabolic disease HIV-infected. Kidney Int 2010;78:478-85.
treatment. Nat Rev Nephrol 2015;11:150-60. in HIV infection. Lancet Infect Dis 2013; 22. Wyatt CM, Schwartz GJ, Owino
5. Notice to readers: final 2015 reports 13:964-75. Ong’or W, et al. Estimating kidney func-
of nationally notifiable infectious diseases 14. Calvo M, Martinez E. Update on met- tion in HIV-infected adults in Kenya: com-
and conditions. MMWR Morb Mortal abolic issues in HIV patients. Curr Opin parison to a direct measure of glomerular
Wkly Rep 2016;65:1306-21. HIV AIDS 2014;9:332-9. filtration rate by iohexol clearance. PLoS
6. Fabian J, Naicker S. HIV and kidney 15. Ryom L, Mocroft A, Kirk O, et al. As- One 2013;8(8):e69601.
disease in sub-Saharan Africa. Nat Rev sociation between antiretroviral exposure 23. Mocroft A, Ryom L, Reiss P, et al.
Nephrol 2009;5:591-8. and renal impairment among HIV-posi- A comparison of estimated glomerular
7. Fox MP, Rosen S, Geldsetzer P, Bär- tive persons with normal baseline renal filtration rates using Cockcroft-Gault and
nighausen T, Negussie E, Beanland R. function: the D:A:D study. J Infect Dis the Chronic Kidney Disease Epidemiology
Interventions to improve the rate or tim- 2013;207:1359-69. Collaboration estimating equations in HIV
ing of initiation of antiretroviral therapy 16. Franceschini N, Napravnik S, Eron JJ infection. HIV Med 2014;15:144-52.
for HIV in sub-Saharan Africa: meta-analy- Jr, Szczech LA, Finn WF. Incidence and 24. Lee FJ, Carr A. Tolerability of HIV in-
ses of effectiveness. J Int AIDS Soc 2016; etiology of acute renal failure among am- tegrase inhibitors. Curr Opin HIV AIDS
19:20888. bulatory HIV-infected patients. Kidney Int 2012;7:422-8.
8. Henry J. Kaiser Family Foundation. 2005;67:1526-31. 25. German P, Liu HC, Szwarcberg J, et al.
The HIV/AIDS epidemic in the United States 17. Cohen SD, Chawla LS, Kimmel PL. Effect of cobicistat on glomerular filtra-
(https://w ww.kff.org/h ivaids/f act-sheet/ Acute kidney injury in patients with hu- tion rate in subjects with normal and im-
the-hivaids-epidemic-in-the-united-states man immunodeficiency virus infection. paired renal function. J Acquir Immune
-the-basics/). Curr Opin Crit Care 2008;14:647-53. Defic Syndr 2012;61:32-40.
2372 n engl j med 377;24 nejm.org December 14, 2017
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
HIV-Associated Kidney Diseases
26. Sreepada Rao TK, Filippone EJ, Nicas- 41. Beckerman P, Bi-Karchin J, Park ASD, update on antiretroviral medications for
tri AD, et al. Associated focal and segmen- et al. Transgenic expression of human nephrologists. Clin J Am Soc Nephrol
tal glomerulosclerosis in the acquired im- APOL1 risk variants in podocytes induces 2006;1:117-29.
munodeficiency syndrome. N Engl J Med kidney disease in mice. Nat Med 2017;23: 56. Eustace JA, Nuermberger E, Choi M,
1984;310:669-73. 429-38. Scheel PJ Jr, Moore R, Briggs WA. Cohort
27. Pardo V, Aldana M, Colton RM, et al. 42. Sauter D, Kirchhoff F. HIV replication: study of the treatment of severe HIV-asso-
Glomerular lesions in the acquired immu- a game of hide and sense. Curr Opin HIV ciated nephropathy with corticosteroids.
nodeficiency syndrome. Ann Intern Med AIDS 2016;11:173-81. Kidney Int 2000;58:1253-60.
1984;101:429-34. 43. Nichols B, Jog P, Lee JH, et al. Innate 57. Bird JE, Durham SK, Giancarli MR, et
28. Gardenswartz MH, Lerner CW, Selig- immunity pathways regulate the nephrop- al. Captopril prevents nephropathy in HIV-
son GR, et al. Renal disease in patients athy gene Apolipoprotein L1. Kidney Int transgenic mice. J Am Soc Nephrol 1998;
with AIDS: a clinicopathologic study. Clin 2015;87:332-42. 9:1441-7.
Nephrol 1984;21:197-204. 44. Hayek SS, Koh KH, Grams ME, et al. 58. Kumar D, Plagov A, Yadav I, et al. In-
29. Heymann J, Winkler CA, Hoek M, A tripartite complex of suPAR, APOL1 hibition of renin activity slows down the
Susztak K, Kopp JB. Therapeutics for risk variants and αvβ3 integrin on podo- progression of HIV-associated nephropa-
APOL1 nephropathies: putting out the cytes mediates chronic kidney disease. thy. Am J Physiol Renal Physiol 2012;303:
fire in the podocyte. Nephrol Dial Trans- Nat Med 2017;23:945-53. F711-F720.
plant 2017;32:Suppl 1:i65-i70. 45. Barisoni L, Schnaper HW, Kopp JB. 59. Campos P, Ortiz A, Soto K. HIV and
30. Dijkman HB, Weening JJ, Smeets B, A proposed taxonomy for the podocytop- kidney diseases: 35 years of history and
et al. Proliferating cells in HIV and pami- athies: a reassessment of the primary ne- consequences. Clin Kidney J 2016;9:772-
dronate-associated collapsing focal seg- phrotic diseases. Clin J Am Soc Nephrol 81.
mental glomerulosclerosis are parietal epi- 2007;2:529-42. 60. Wyatt CM, Morgello S, Katz-Malamed
thelial cells. Kidney Int 2006;70:338-44. 46. Olabisi OA, Zhang JY, VerPlank L, R, et al. The spectrum of kidney disease
31. Mallipattu SK, Wyatt CM, He JC. The et al. APOL1 kidney disease risk variants in patients with AIDS in the era of antiret-
new epidemiology of HIV-related kidney cause cytotoxicity by depleting cellular roviral therapy. Kidney Int 2009;75:428-34.
disease. J AIDS Clin Res 2012;Suppl 4:001. potassium and inducing stress-activated 61. Kimmel PL, Phillips TM, Ferreira-
32. Razzak Chaudhary S, Workeneh BT, protein kinases. Proc Natl Acad Sci U S A Centeno A, Farkas-Szallasi T, Abraham
Montez-Rath ME, Zolopa AR, Klotman 2016;113:830-7. AA, Garrett CT. Idiotypic IgA nephropa-
PE, Winkelmayer WC. Trends in the out- 47. Ma L, Chou JW, Snipes JA, et al. thy in patients with human immunodefi-
comes of end-stage renal disease second- APOL1 renal-risk variants induce mito- ciency virus infection. N Engl J Med 1992;
ary to human immunodeficiency virus- chondrial dysfunction. J Am Soc Nephrol 327:702-6.
associated nephropathy. Nephrol Dial 2017;28:1093-105. 62. Kimmel PL, Phillips TM, Ferreira-
Transplant 2015;30:1734-40. 48. Fine DM, Wasser WG, Estrella MM, Centeno A, Farkas-Szallasi T, Abraham AA,
33. Genovese G, Friedman DJ, Ross MD, et al. APOL1 risk variants predict histopa- Garrett CT. HIV-associated immune-
et al. Association of trypanolytic ApoL1 thology and progression to ESRD in HIV- mediated renal disease. Kidney Int 1993;
variants with kidney disease in African related kidney disease. J Am Soc Nephrol 44:1327-40.
Americans. Science 2010;329:841-5. 2012;23:343-50. 63. Booth JW, Hamzah L, Jose S, et al.
34. Tzur S, Rosset S, Shemer R, et al. Mis- 49. Nobakht E, Cohen SD, Rosenberg AZ, Clinical characteristics and outcomes of
sense mutations in the APOL1 gene are Kimmel PL. HIV-associated immune com- HIV-associated immune complex kidney
highly associated with end stage kidney plex kidney disease. Nat Rev Nephrol 2016; disease. Nephrol Dial Transplant 2016;31:
disease risk previously attributed to the 12:291-300. 2099-107.
MYH9 gene. Hum Genet 2010;128:345-50. 50. Avettand-Fenoël V, Rouzioux C, Legen- 64. Ghrenassia E, Martis N, Boyer J, Burel-
35. Raper J, Fung R, Ghiso J, Nussenzweig dre C, Canaud G. HIV infection in the na- Vandenbos F, Mekinian A, Coppo P. The
V, Tomlinson S. Characterization of a novel tive and allograft kidney: implications for diffuse infiltrative lymphocytosis syn-
trypanosome lytic factor from human se- management, diagnosis and transplanta- drome (DILS): a comprehensive review.
rum. Infect Immun 1999;67:1910-6. tion. Transplantation 2017;101:2003-8. J Autoimmun 2015;59:19-25.
36. Cooper A, Ilboudo H, Alibu VP, et al. 51. Lucas GM, Ross MJ, Stock PG, et al. 65. Cohen SD, Kimmel PL. Renal biopsy is
APOL1 renal risk variants have contrast- Clinical practice guideline for the man- necessary for the diagnosis of HIV-associ-
ing resistance and susceptibility associa- agement of chronic kidney disease in pa- ated renal diseases. Nat Clin Pract Nephrol
tions with African trypanosomiasis. Elife tients infected with HIV: 2014 update by 2009;5:22-3.
2017;6:e25461. the HIV Medicine Association of the In- 66. Becker S, Fusco G, Fusco J, et al. HIV-
37. Kopp JB, Nelson GW, Sampath K, et al. fectious Diseases Society of America. Clin associated thrombotic microangiopathy in
APOL1 genetic variants in focal segmen- Infect Dis 2014;59(9):e96-e138. the era of highly active antiretroviral ther-
tal glomerulosclerosis and HIV-associated 52. Vital signs: HIV prevention through apy: an observational study. Clin Infect Dis
nephropathy. J Am Soc Nephrol 2011;22: care and treatment — United States. 2004;39:Suppl 5:S267-75.
2129-37. MMWR Morb Mortal Wkly Rep 2011;60: 67. Gervasoni C, Ridolfo AL, Vaccarezza
38. Kasembeli AN, Duarte R, Ramsay M, 1618-23. M, et al. Thrombotic microangiopathy in
et al. APOL1 risk variants are strongly as- 53. Stöhr W, Reid A, Walker AS, et al. patients with acquired immunodeficiency
sociated with HIV-associated nephropa- Glomerular dysfunction and associated syndrome before and during the era of in-
thy in black South Africans. J Am Soc risk factors over 4-5 years following anti- troduction of highly active antiretroviral
Nephrol 2015;26:2882-90. retroviral therapy initiation in Africa. Anti- therapy. Clin Infect Dis 2002;35:1534-40.
39. Kruzel-Davila E, Shemer R, Ofir A, vir Ther 2011;16:1011-20. 68. del Arco A, Martinez MA, Peña JM, et
et al. APOL1-mediated cell injury involves 54. Mpondo BC, Kalluvya SE, Peck RN, al. Thrombotic thrombocytopenic purpura
disruption of conserved trafficking pro- et al. Impact of antiretroviral therapy on associated with human immunodeficiency
cesses. J Am Soc Nephrol 2017;28:1117-30. renal function among HIV-infected Tan- virus infection: demonstration of p24 an-
40. Fu Y, Zhu JY, Richman A, et al. APOL1- zanian adults: a retrospective cohort tigen in endothelial cells. Clin Infect Dis
G1 in nephrocytes induces hypertrophy study. PLoS One 2014;9(2):e89573. 1993;17:360-3.
and accelerates cell death. J Am Soc Nephrol 55. Berns JS, Kasbekar N. Highly active 69. Izzedine H, Deray G. The nephrologist
2017;28:1106-16. antiretroviral therapy and the kidney: an in the HAART era. AIDS 2007;21:409-21.
n engl j med 377;24 nejm.org December 14, 2017 2373
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
HIV-Associated Kidney Diseases
70. Jin A, Boroujerdi-Rad L, Shah G, Chen Schafer JJ. Tenofovir alafenamide. Ann ease in a large cohort of HIV-1 infected
JL. Thrombotic microangiopathy and hu- Pharmacother 2016;50:942-52. individuals initiating antiretroviral ther-
man immunodeficiency virus in the era 78. Daugas E, Plaisier E, Boffa JJ, et al. apy in routine care. AIDS 2012;26:1907-
of eculizumab. Clin Kidney J 2016;9:576- Acute renal failure associated with immune 15.
9. restoration inflammatory syndrome. Nat 85. Soleymanian T, Raman S, Shannaq FN,
71. Meehan SM, Kim L, Chang A. A spec- Clin Pract Nephrol 2006;2:594-8. et al. Survival and morbidity of HIV pa-
trum of morphologic lesions of focal seg- 79. Peralta C, Scherzer R, Grunfeld C, et al. tients on hemodialysis and peritoneal di-
mental glomerulosclerosis by Columbia Urinary biomarkers of kidney injury are alysis: one center’s experience and review
criteria in human immunodeficiency virus associated with all-cause mortality in the of the literature. Int Urol Nephrol 2006;
infection. Virchows Arch 2012;460:429- Women’s Interagency HIV Study (WIHS). 38:331-8.
35. HIV Med 2014;15:291-300. 86. Farzadegan H, Ford D, Malan M, Mas-
72. Izzedine H, Harris M, Perazella MA. 80. Shlipak MG, Scherzer R, Abraham A, ters B, Scheel PJ Jr. HIV-1 survival kinetics
The nephrotoxic effects of HAART. Nat et al. Urinary markers of kidney injury in peritoneal dialysis effluent. Kidney Int
Rev Nephrol 2009;5:563-73. and kidney function decline in HIV-infected 1996;50:1659-62.
73. Mocroft A, Lundgren JD, Ross M, et al. women. J Acquir Immune Defic Syndr 2012; 87. Stock PG, Barin B, Murphy B, et al.
Development and validation of a risk score 61:565-73. Outcomes of kidney transplantation in HIV-
for chronic kidney disease in HIV infec- 81. Jotwani V, Scherzer R, Estrella MM, infected recipients. N Engl J Med 2010;
tion using prospective cohort data from et al. HIV infection, tenofovir disoproxil 363:2004-14.
the D:A:D study. PLoS Med 2015;12(3): fumarate, and urine alpha1-microglobu- 88. Sawinski D, Bloom RD. Current status
e1001809. lin: a cross-sectional analysis in the Multi- of kidney transplantation in HIV-infected
74. Scherzer R, Gandhi M, Estrella MM, center AIDS Cohort Study. Am J Kidney patients. Curr Opin Nephrol Hypertens
et al. A chronic kidney disease risk score Dis 2016;68:571-81. 2014;23:619-24.
to determine tenofovir safety in a prospec- 82. Siedner MJ, Atta MG, Lucas GM, Pera- 89. Freedman BI, Julian BA. Should kid-
tive cohort of HIV-positive male veterans. zella MA, Fine DM. Poor validity of urine ney donors be genotyped for APOL1 risk
AIDS 2014;28:1289-95. dipstick as a screening tool for protein- alleles? Kidney Int 2015;87:671-3.
75. Mallipattu SK, Salem F, Wyatt CM. uria in HIV-positive patients. J Acquir Im- 90. Boyarsky BJ, Durand CM, Palella FJ Jr,
The changing epidemiology of HIV-related mune Defic Syndr 2008;47:261-3. Segev DL. Challenges and clinical decision-
chronic kidney disease in the era of anti- 83. Kidney Disease: Improving Global making in HIV-to-HIV transplantation:
retroviral therapy. Kidney Int 2014; 86: Outcomes (KDIGO) CKD Work Group. insights from the HIV literature. Am J
259-65. KDIGO clinical practice guideline for the Transplant 2015;15:2023-30.
76. Wyatt CM. Will a new tenofovir pro- evaluation and management of chronic 91. Muller E, Barday Z, Mendelson M,
drug for the treatment of HIV reduce the kidney disease. Kidney Int Suppl 2013;3: Kahn D. HIV-positive–to–HIV-positive kid-
risk of nephrotoxicity? Kidney Int 2016; 1-150. ney transplantation — results at 3 to 5 years.
89:5-6. 84. Kalayjian RC, Lau B, Mechekano RN, N Engl J Med 2015;372:613-20.
77. Gibson AK, Shah BM, Nambiar PH, et al. Risk factors for chronic kidney dis- Copyright © 2017 Massachusetts Medical Society.
images in clinical medicine
The Journal welcomes consideration of new submissions for Images in Clinical
Medicine. Instructions for authors and procedures for submissions can be found
on the Journal’s website at NEJM.org. At the discretion of the editor, images that
are accepted for publication may appear in the print version of the Journal,
the electronic version, or both.
2374 n engl j med 377;24 nejm.org December 14, 2017
The New England Journal of Medicine
Downloaded from nejm.org by RAMYASRI KODALI on January 4, 2018. For personal use only. No other uses without permission.
Copyright © 2017 Massachusetts Medical Society. All rights reserved.
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- ACTIVITY NO - 6 - Parasitology - Parasitic HelminthesДокумент8 страницACTIVITY NO - 6 - Parasitology - Parasitic HelminthesMichelle HutamaresОценок пока нет
- 2 Pioneers and Milestones in The Field of Orthodontics and Orthognathic Surgery 2014 Orthognathic SurgeryДокумент43 страницы2 Pioneers and Milestones in The Field of Orthodontics and Orthognathic Surgery 2014 Orthognathic SurgeryiweourvgiuОценок пока нет
- AINSHAMS - Master of DermatologyДокумент84 страницыAINSHAMS - Master of DermatologyMuhammad SammyОценок пока нет
- Blood BankДокумент15 страницBlood BankAnsari ShariqОценок пока нет
- ODB - Nursing Thera 2 (NLE)Документ2 страницыODB - Nursing Thera 2 (NLE)AP Pride Slear DeveraОценок пока нет
- Health Appraisal QuestionnaireДокумент5 страницHealth Appraisal QuestionnaireSteveОценок пока нет
- Acute Kidney InjuryДокумент44 страницыAcute Kidney InjurySuci MayveraОценок пока нет
- Dialysis Event Surveillance ManualДокумент56 страницDialysis Event Surveillance ManualAnitha SОценок пока нет
- Sharad Coaching: Sense Organs 1 Marks QuestionsДокумент8 страницSharad Coaching: Sense Organs 1 Marks QuestionsArnav AgarwalОценок пока нет
- Retrospective Study - The Presence of Malassezia in Feline Skin Biopsies. A Clinicopathological Study (Pages 7-14)Документ8 страницRetrospective Study - The Presence of Malassezia in Feline Skin Biopsies. A Clinicopathological Study (Pages 7-14)jenОценок пока нет
- Intrathyroidal Lymphoepithelial (Branchial) Cyst: Diagnostic and Management Challenge of A Rare EntityДокумент5 страницIntrathyroidal Lymphoepithelial (Branchial) Cyst: Diagnostic and Management Challenge of A Rare EntityMeliОценок пока нет
- Kapita Selekta ImunologiДокумент29 страницKapita Selekta ImunologiAsyha KantifaОценок пока нет
- Histochemistry Chapter 5Документ48 страницHistochemistry Chapter 5Zelalem DejazmachОценок пока нет
- Hormone Therapy and Castration Resistance of Prostate CancerДокумент420 страницHormone Therapy and Castration Resistance of Prostate CancerDiego RamosОценок пока нет
- Drug Bug TableДокумент1 страницаDrug Bug TableLes SangaОценок пока нет
- Acog Gestacion Multiple 2016 PDFДокумент16 страницAcog Gestacion Multiple 2016 PDFRicardo QuintanaОценок пока нет
- The Radiology Assistant - US of The GI Tract - TechniqueДокумент7 страницThe Radiology Assistant - US of The GI Tract - Techniqueابو عبد الرحمنОценок пока нет
- CSU Acute Pain Scale KittenДокумент1 страницаCSU Acute Pain Scale KittenAna Ximena DíazОценок пока нет
- Case Study 11Документ28 страницCase Study 11api-301883277Оценок пока нет
- Mediastinum Tumors PDFДокумент11 страницMediastinum Tumors PDFJose SirittОценок пока нет
- CPG Management of Autism Spectrum Disorder in Children and AdolescentsДокумент76 страницCPG Management of Autism Spectrum Disorder in Children and AdolescentsMuhammad Siddiq100% (1)
- 2Документ2 страницы2Lloyd VillegasОценок пока нет
- Carcinoma Pancreas: Risk Factors: (A) Demographic FactorsДокумент4 страницыCarcinoma Pancreas: Risk Factors: (A) Demographic FactorsSakthi Annamalai.cОценок пока нет
- Herbal Formulation Considerations For Autoimmune DisordersДокумент26 страницHerbal Formulation Considerations For Autoimmune Disordershitesh mendirattaОценок пока нет
- JohnHopkins Post Covid ClinicДокумент7 страницJohnHopkins Post Covid ClinicWalter ReyesОценок пока нет
- Work Immersion 2023Документ12 страницWork Immersion 2023Cuasay, Vernelle Stephanie De guzmanОценок пока нет
- Oral Anatomy DefinitionДокумент17 страницOral Anatomy DefinitionJoe AjibadeОценок пока нет
- BernottiДокумент44 страницыBernottiAlexandra Alexandra100% (2)
- How Microorganism Cause DiseaseДокумент16 страницHow Microorganism Cause DiseaseMARTINEZ JUSTINEОценок пока нет
- Nutrition - TPN (Basics)Документ37 страницNutrition - TPN (Basics)Giorgi BradОценок пока нет