Академический Документы
Профессиональный Документы
Культура Документы
chm432 - 4
Загружено:
Nur AthirahАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
chm432 - 4
Загружено:
Nur AthirahАвторское право:
Доступные форматы
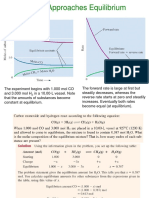
Introduction
The dependence of a reaction on concentration and order of reaction isdescribed by a
rate law that has the following form:
Rate = k [A]x[B]y[C]z
where k is the rate of constant, x is the order of a reaction with respect to reactant A, y
is the order of reaction with respect to reactant B,z is the order of reaction with
respect to reactant C and [ ] represent concentration in moles per litre. The sum of x,y
and z is known as the overall order of reaction. Even though temperature is not
included in the rate law, its plays an important role in the rate of reaction so it must be
held constant during a kinetic experiment. The rate of a reaction must be determined
experimentally and it cannot be determined by looking at the balanced chemical
equation.
In order to determine the speed of the reaction we are going to focus on the
permanganate and simply equate the speed with the time it takes to use up the
permanganate. The method of initial rates will be used to find the exponents x and y.
this method involves measuringa nd comparing the initial rates of reaction when
different initial concentrations are used. Teh initial rate for each being the potassium
permanganate concentration divided by time. There is an assumption made here that
is not always valid: we will be measuring tfinal to determine each rate. For experiments
1 and 2 we can write the rate expressions as :
Rate1 = k [KMnO4]1X[H2C2O4]1y Rate1 = [KMnO4]1/ t1
Rate2 = k [KMnO4]2X[H2C2O4]2y Rate2 = [KMnO4]2 / t2
Taking the ratio of the two rates:
Rate1 / Rate2 = ([KMnO4]1 / [KMnO4]2)X
In these reactions [H2C2O4]1 = [H2C2O4]2, and thus can be cancelled out.
Thus, we can detremine x. In similar manner, y, the order reaction with respect to
oxalic acid can be determined if we compare experiments 1 and 3.
Procedures
Three burettes contained KMnO4, H2C2O4 and distilled water was set up. Each of
burettes was labelled. The exact molarities of KMnO4 and H2C2O4 was recorded. The
required amount of H2C2O4 and water (if any) was placed into a washed and dried
conical flask according to Table 4.1. the amounts was dictated by the experiment that
was doing. If the amount overshoot, we have to start again to get the exact amount as
this lab is very dependent on dispensing the exact quantities.
Table 4.1 : Volume of reagents required
Reagents Exp 1 Exp 2 Exp 3
cm3 cm3 cm3
H2C2O4 20.00 20.00 10.00
KMnO4 10.00 5.00 10.00
H2O 0.00 5.00 10.00
The required amount of KMnO4 was placed into a test tube. The KMnO4 solution was
added to the oxalic acid and the timing started when the permanganate tube was
emptied. The mixture was swirled to mix it and continued swirling until the solution
turns to light yellow. The timer was stopped and the time it actually took for the
reaction to take place was recorded. The step was repeated with a second and third
trial. The average reaction time of those three was recorded. Repeat all the steps
above for expeiment 2 and 3. The rate for each of the three experiments was
determined. The formula was [KMnO4]/taverage. The values of k, x and y were
determined. The full rate equation was
Rate = k [KMnO4]X[H2C2O4]y
Вам также может понравиться
- Exp 4 Kinetics Order of ReactionДокумент8 страницExp 4 Kinetics Order of ReactionNur Fadhilah0% (1)
- Physical Chemistry 1 (CHM 471) : Faculty of Applied Sciences Laboratory ReportДокумент10 страницPhysical Chemistry 1 (CHM 471) : Faculty of Applied Sciences Laboratory ReportHusna Insyirah Bt SamadОценок пока нет
- 112 Experiment 3Документ3 страницы112 Experiment 3Amal ..Оценок пока нет
- Experiment 2: Kinetics of The Reaction Between Permanganate and Oxalic AcidДокумент4 страницыExperiment 2: Kinetics of The Reaction Between Permanganate and Oxalic AcidMaryNicoleDatlanginОценок пока нет
- Experiment 4 CHM476Документ10 страницExperiment 4 CHM476Hazwan Hamim100% (1)
- Exp 4-Rate LawДокумент9 страницExp 4-Rate Lawtkjing100% (1)
- Oxidation of Isopropanol by Chromium (Vi) ReportДокумент12 страницOxidation of Isopropanol by Chromium (Vi) ReportGideonОценок пока нет
- Exp 4 Kinetics: Order of ReactionДокумент8 страницExp 4 Kinetics: Order of ReactionMuhammad Amirul AfifiОценок пока нет
- Kinetics 2Документ5 страницKinetics 2refaq AhmadОценок пока нет
- Kinetics Order of ReactionДокумент6 страницKinetics Order of ReactionFath Bond0% (3)
- CRE I Assignment 5Документ2 страницыCRE I Assignment 5Sumit KatkarОценок пока нет
- Catalyzed Decomposition of Hydrogen PeroxideДокумент5 страницCatalyzed Decomposition of Hydrogen PeroxideDennis WrinОценок пока нет
- Experiment 2K3Документ10 страницExperiment 2K3Inkiru N. BernardОценок пока нет
- Formal Report in Analytical ChemistryДокумент5 страницFormal Report in Analytical ChemistryJohn Rally Jr FilamorОценок пока нет
- Chapter 6Документ17 страницChapter 6helloblarg100% (3)
- Iodination of Acetone KineticsДокумент4 страницыIodination of Acetone Kineticsinstrutech0% (2)
- Suggested Solutions To Tutorial 6 - Reaction Kinetics Self-CheckДокумент12 страницSuggested Solutions To Tutorial 6 - Reaction Kinetics Self-CheckDomОценок пока нет
- Sample Kinetics ExperimentДокумент7 страницSample Kinetics ExperimentVenus PondevidaОценок пока нет
- Solubility Equilibrium of Calcium HydroxideДокумент6 страницSolubility Equilibrium of Calcium HydroxideMartin Cirio100% (1)
- Reaction KineticsДокумент7 страницReaction Kineticsjathan160% (1)
- Lab #4 - FinalДокумент8 страницLab #4 - FinalEmmaОценок пока нет
- 2 - Homogenous CatalysisДокумент3 страницы2 - Homogenous CatalysisVo Trung Kien B2100780Оценок пока нет
- Experiment 15: Chemical KineticsДокумент26 страницExperiment 15: Chemical KineticsLutendo Assurance MadzivhaaОценок пока нет
- Tbutyl Report PDFДокумент20 страницTbutyl Report PDFEhsan RahmanОценок пока нет
- CH17 (1,2,3) Kinetics CHM152Документ49 страницCH17 (1,2,3) Kinetics CHM152KiranОценок пока нет
- 471 Homework 1 2008Документ3 страницы471 Homework 1 2008HungDoОценок пока нет
- 112 Experiment 4Документ3 страницы112 Experiment 4Abhishek KunduОценок пока нет
- Research Article of Equilibrium Constant in Esterification ReactionДокумент4 страницыResearch Article of Equilibrium Constant in Esterification ReactionViha Ancillia25% (4)
- Activation EnergyДокумент1 страницаActivation EnergyRahul ChoudharyОценок пока нет
- Chemistry 112 Dye Kinetics Laboratory 2011Документ4 страницыChemistry 112 Dye Kinetics Laboratory 2011Lavenia Alou MagnoОценок пока нет
- Unit 2 Linear Simultaneous Algebraic EquationДокумент2 страницыUnit 2 Linear Simultaneous Algebraic EquationsaravananarajuОценок пока нет
- The Kinetic Study of The IodinationДокумент6 страницThe Kinetic Study of The IodinationsamОценок пока нет
- Batch Adiabatic ReactorДокумент6 страницBatch Adiabatic ReactorHarsh TekriwalОценок пока нет
- Chemical Equilibrium-UAPДокумент26 страницChemical Equilibrium-UAPMd. Mujahid HasanОценок пока нет
- Discussions: TFR AOДокумент3 страницыDiscussions: TFR AOahmad pidotОценок пока нет
- Chm432 Expt 4Документ9 страницChm432 Expt 4Ievana InsyirahОценок пока нет
- AP Chemistry - Kinetics of A Reaction LabДокумент8 страницAP Chemistry - Kinetics of A Reaction LabJonathan Chen50% (2)
- Exp 6 Determination of The Molar Volume of A Gas and The Universal Gas ConstantДокумент5 страницExp 6 Determination of The Molar Volume of A Gas and The Universal Gas ConstantMerrene Bright Divino JudanОценок пока нет
- P4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperatureДокумент7 страницP4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperaturejayaprinaОценок пока нет
- Saturday X-Tra X-Sheet: 16 Rates of Reaction: Key ConceptsДокумент6 страницSaturday X-Tra X-Sheet: 16 Rates of Reaction: Key ConceptsKingford MwesoОценок пока нет
- Experiment 1 Phy Chem 1Документ7 страницExperiment 1 Phy Chem 1Junard LandinginОценок пока нет
- Crystal VioletДокумент10 страницCrystal VioletTaniya GuptaОценок пока нет
- SCH 103 - Physical and General ChemistryДокумент65 страницSCH 103 - Physical and General ChemistrySamwel OchokОценок пока нет
- Chemical Equilibrium: CHE 195 Process ChemistryДокумент25 страницChemical Equilibrium: CHE 195 Process ChemistryMohd Shahrul Nizam SallehОценок пока нет
- Lab Report: Investigation of First Order Response Kinetics: I.AbstractДокумент5 страницLab Report: Investigation of First Order Response Kinetics: I.AbstractHoàng Thu HằngОценок пока нет
- Lab #4Документ8 страницLab #4EmmaОценок пока нет
- CH4002 L12-16 2017 KH Chemical Kinetics IntroductionДокумент56 страницCH4002 L12-16 2017 KH Chemical Kinetics Introductionjesuslovers3000Оценок пока нет
- CH15Документ9 страницCH15Criselle VillarosaОценок пока нет
- Catalytic Oxidation of Benzene To Maleic Anhydride in A Continuous Stirred Tank ReactorДокумент7 страницCatalytic Oxidation of Benzene To Maleic Anhydride in A Continuous Stirred Tank ReactorMirko GraneseОценок пока нет
- Orsms l07 & l08 - Fall 2022Документ57 страницOrsms l07 & l08 - Fall 2022Yousef EssamОценок пока нет
- Experiment FourДокумент10 страницExperiment FourYana SyafriyanaОценок пока нет
- Postlab Expt2 CaminongДокумент7 страницPostlab Expt2 CaminongAdrian Sean CaminongОценок пока нет
- Catalytic Production of Cyclohexane-Oxime Via Cyclohexanone AmmoximationДокумент9 страницCatalytic Production of Cyclohexane-Oxime Via Cyclohexanone AmmoximationKevin ThomasОценок пока нет
- Chemical Kinetics LectureДокумент21 страницаChemical Kinetics LectureMohamed MegahedОценок пока нет
- Lab 10-Batch ReactorДокумент22 страницыLab 10-Batch Reactorniraj_bairagiОценок пока нет
- Try This For Lab Report #5Документ16 страницTry This For Lab Report #5thuy duongОценок пока нет
- Physical CHemistry:Iodinization of AcetoneДокумент4 страницыPhysical CHemistry:Iodinization of AcetoneLevy Medina TrayaОценок пока нет
- chm457 4Документ3 страницыchm457 4Nur Athirah0% (1)
- Chm421 LabДокумент3 страницыChm421 LabNur AthirahОценок пока нет
- chm457 2Документ3 страницыchm457 2Nur AthirahОценок пока нет
- Elc650 Email GuidelinesДокумент2 страницыElc650 Email GuidelinesNur Athirah50% (2)
- References: Mistry - (Smith) /chapter - 24: - Carbonyl - Condensation - Reactions/24.8: - The - Michael - ReactionДокумент2 страницыReferences: Mistry - (Smith) /chapter - 24: - Carbonyl - Condensation - Reactions/24.8: - The - Michael - ReactionNur AthirahОценок пока нет
- Inorganic Chemistry: Experiment 7: Measurement of Physical Properties and Isomerism of ComplexesДокумент4 страницыInorganic Chemistry: Experiment 7: Measurement of Physical Properties and Isomerism of ComplexesNur AthirahОценок пока нет
- Experiment 5: Preparation of An, Unsaturated Ketone Via Michael Addition and Aldol Condensation ReactionsДокумент10 страницExperiment 5: Preparation of An, Unsaturated Ketone Via Michael Addition and Aldol Condensation ReactionsNur AthirahОценок пока нет
- Cocl 6 NH Orange-Yellow Cocl 5 NH H O Red Cocl 5 NH Purple Cocl 4 NH GreenДокумент3 страницыCocl 6 NH Orange-Yellow Cocl 5 NH H O Red Cocl 5 NH Purple Cocl 4 NH GreenNur AthirahОценок пока нет
- Title: Preparation of 4-MethylcyclohexeneДокумент7 страницTitle: Preparation of 4-MethylcyclohexeneNur AthirahОценок пока нет
- ExpДокумент6 страницExpNur AthirahОценок пока нет
- Obesity: Term Paper OnДокумент13 страницObesity: Term Paper OnNur AthirahОценок пока нет
- Exp 1Документ5 страницExp 1Nur AthirahОценок пока нет
- Obesity: Term Paper OnДокумент13 страницObesity: Term Paper OnNur AthirahОценок пока нет
- Experiment 1 Isolation of Caffeine From A Tea BagДокумент1 страницаExperiment 1 Isolation of Caffeine From A Tea BagNur AthirahОценок пока нет
- 1.0 Chemical BondingДокумент42 страницы1.0 Chemical BondingNur AthirahОценок пока нет
- Provision of EPCI Services For EPCIC Sidayu Wellhead Platforms and Pipelines Sidayu Field DevelopmentДокумент7 страницProvision of EPCI Services For EPCIC Sidayu Wellhead Platforms and Pipelines Sidayu Field Developmentriandi100% (1)
- Surface TensionДокумент13 страницSurface TensionElizebeth GОценок пока нет
- Eldritch HighДокумент39 страницEldritch Highteam_moОценок пока нет
- Honeycomb Kevlar 49 (Hexcel)Документ3 страницыHoneycomb Kevlar 49 (Hexcel)Julia GarciaОценок пока нет
- Flight Vehicle Design:: Example 2 (Uav)Документ43 страницыFlight Vehicle Design:: Example 2 (Uav)Anmol KumarОценок пока нет
- Keiilf: Training ManualДокумент53 страницыKeiilf: Training ManualGary GouveiaОценок пока нет
- Fyp-Hydraulic Brakes CompleteДокумент32 страницыFyp-Hydraulic Brakes CompleteRishabh JainОценок пока нет
- Math AA SL P 1 Marks SchemeДокумент6 страницMath AA SL P 1 Marks SchemeMrin GhoshОценок пока нет
- Harmonics PatternsДокумент4 страницыHarmonics PatternsIzzadAfif1990Оценок пока нет
- Liver: Anatomy & FunctionsДокумент18 страницLiver: Anatomy & FunctionsDR NARENDRAОценок пока нет
- Guia de CondensadoresДокумент193 страницыGuia de CondensadoresPaola Segura CorreaОценок пока нет
- Douluo Dalu Volume 05 - Star Dou Forest PDFДокумент141 страницаDouluo Dalu Volume 05 - Star Dou Forest PDFRay Joseph LealОценок пока нет
- Native Data Sheet Asme b73.1Документ4 страницыNative Data Sheet Asme b73.1Akhmad Faruq Alhikami100% (1)
- 9 5 - 358 362 PDFДокумент5 страниц9 5 - 358 362 PDFمالك مناصرةОценок пока нет
- S TR GEN ID (Component Marking) (Rev 3 2009) - AN Marked UpДокумент6 страницS TR GEN ID (Component Marking) (Rev 3 2009) - AN Marked UpsnclgsraoОценок пока нет
- Fines Reduction Project at Wendling Bowser QuarryДокумент2 страницыFines Reduction Project at Wendling Bowser QuarryMarcos Antonio ParoliniОценок пока нет
- DR PDFДокумент252 страницыDR PDFa_ouchar0% (1)
- Sika - Bitumen: Bitumen Emulsion Waterproof & Protective CoatingДокумент3 страницыSika - Bitumen: Bitumen Emulsion Waterproof & Protective Coatingdinu69inОценок пока нет
- Plastics and Polymer EngineeringДокумент4 страницыPlastics and Polymer Engineeringsuranjana26Оценок пока нет
- MTH100Документ3 страницыMTH100Syed Abdul Mussaver ShahОценок пока нет
- Anxiety Disorders - Causes, Types, Symptoms, & TreatmentsДокумент5 страницAnxiety Disorders - Causes, Types, Symptoms, & Treatmentsrehaan662Оценок пока нет
- Arbor APS STT Unit 01 Design Basics 25 Jan2018Документ31 страницаArbor APS STT Unit 01 Design Basics 25 Jan2018masterlinh2008Оценок пока нет
- Determination of Drop-Impact Resistance of Plastic BottlesДокумент11 страницDetermination of Drop-Impact Resistance of Plastic BottlesAndres BrañaОценок пока нет
- FemДокумент4 страницыFemAditya SharmaОценок пока нет
- Industrial Artificial Intelligence For Industry 4.0-Based Manufacturing SystemsДокумент5 страницIndustrial Artificial Intelligence For Industry 4.0-Based Manufacturing SystemsMuhammad HaziqОценок пока нет
- Owner'S Manual: 2023 Chassis CabДокумент444 страницыOwner'S Manual: 2023 Chassis CabDmitry DimasОценок пока нет
- Fully Automatic Coffee Machine - Slimissimo - IB - SCOTT UK - 2019Документ20 страницFully Automatic Coffee Machine - Slimissimo - IB - SCOTT UK - 2019lazareviciОценок пока нет
- Tuberculosis PowerpointДокумент69 страницTuberculosis PowerpointCeline Villo100% (1)
- DMDWLab Book AnswersДокумент44 страницыDMDWLab Book AnswersNarpat Makwana Pune100% (1)
- Prestige Institute of Management & Research: Guided By:-Submitted By: - Prof. Arpit Loya Sumeet RattanДокумент21 страницаPrestige Institute of Management & Research: Guided By:-Submitted By: - Prof. Arpit Loya Sumeet RattanSumeet700005Оценок пока нет