Академический Документы
Профессиональный Документы
Культура Документы
X
Загружено:
XxxИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
X
Загружено:
XxxАвторское право:
Доступные форматы
Name : Vike Yuniasri
NIM : 21030115120060
Class : International
TASK 1 Chemical Reaction Engineering
From Levenspiel Book 23.1 and 23.6
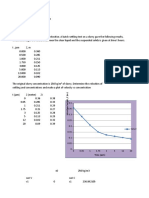
Gaseous A absorbs and reacts with B in liquid according to
in a packed bed under conditions where
At a point in the reactor where pA = 100 Pa and CB = 100 mol/m3 liquid
(a) calculate the rate of reaction in mol/hr . m3 of reactor.
(b) describe the following characteristics of the kinetics:
location of the major resistance (gas film, liquid film, main body of liquid)
behavior in the liquid film (pseudo first-order reaction, instantaneous, second-
order reaction, physical transport) for the following values of reaction rate and
Henry's law constant.
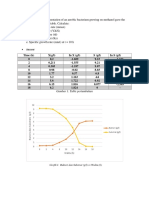
Name : Vike Yuniasri
NIM : 21030115120060
Class : International
Solution:
D k Cb 10 -6 x10x102
23.1 Mh= √ =√ =3.16 x 10-2
kAl 1
Because the Mh value is 3.16 𝑥 10−2 , the value of E is 1
PA 102 mol
-ra = 1 H H = 105 105 =0,009
+ a + a 1
+ 2 (1)+ hr.m3 of reactor
Kag a Kal aE KCB Fl 0,1 10 10(102 )0,01
9.08% 90.82%
mol
(a) Reaction rate = 0.009 hr m3 reactor
(b) Resistance = 90.82% liquid film, 9.08% liquid bulk
Reaction zone: main body of liquid
Behavior in liquid film: physical transport
D k Cb 10-6 x108 x102
23.6 Mh= √ =√ =102
kAl 1
DB CB HA 10-6 x100x1
(Ei)firstguess=1+ =1+ =2
b DA pAi 1 x10-6 x100
Since E first guess < Mh/5, we have instantaneous reaction at L film:
E = El = 2
KAg pA =0.1 x 100=10
KBl 100
CB = x100=104
b 1
kBl
Since kAgpA< Cb, we use Instantaneous Reaction, High Cb
b
mol
-rA'' = kAgpA=10 hr m3 reactor
mol
(a) Reaction rate = 10 reactor
hr m3
(b) Behavior in liquid film: instantaneous reaction
Вам также может понравиться
- Te HEДокумент6 страницTe HEjamieОценок пока нет
- Tutorial 5Документ7 страницTutorial 5Saints Burner ChristopherОценок пока нет
- Geankoplis 12.10-5: Nama: Qasimatul Wasilah Kelas: 3d D4 Tki Nim: 1841420068Документ8 страницGeankoplis 12.10-5: Nama: Qasimatul Wasilah Kelas: 3d D4 Tki Nim: 1841420068CiciОценок пока нет
- Tugas PAP Latihan Soal Bab 7Документ2 страницыTugas PAP Latihan Soal Bab 7IvanaMargaretОценок пока нет
- Debhie Cesilia E - 5213418072 - QUIZ PERPANДокумент12 страницDebhie Cesilia E - 5213418072 - QUIZ PERPANFenita Yuni PratiwiОценок пока нет
- Impor Dimethyl Ether (DME)Документ3 страницыImpor Dimethyl Ether (DME)Savannah Yonita CОценок пока нет
- ChE 195 Problem Set No. 2Документ1 страницаChE 195 Problem Set No. 2Jahz ChannelОценок пока нет
- Something Related To Catalysts.Документ2 страницыSomething Related To Catalysts.Deepro BhattacharyaОценок пока нет
- Flash DrumДокумент14 страницFlash DrumFajar AgumОценок пока нет
- Sample Problem 16 PDFДокумент9 страницSample Problem 16 PDFJoshua Arrojo100% (1)
- Pemisahan WowДокумент11 страницPemisahan WowdianОценок пока нет
- Tutorial Chapter 2 PDFДокумент2 страницыTutorial Chapter 2 PDFKaul PatrickОценок пока нет
- 4 2020 Pap Menara DistilasiДокумент48 страниц4 2020 Pap Menara DistilasiAlwan Al AzharОценок пока нет
- Assignment FINALДокумент67 страницAssignment FINALlaila khanОценок пока нет
- Latihan Soal Packed Bed AbsorberДокумент7 страницLatihan Soal Packed Bed AbsorberAgam Duma Kalista WibowoОценок пока нет
- Thermo Homework 6Документ7 страницThermo Homework 6Danny BoyleОценок пока нет
- Back To School PowerPoint TemplateДокумент12 страницBack To School PowerPoint TemplateRahmasari Nur Setyono0% (1)
- Molar Volume (Rumus)Документ6 страницMolar Volume (Rumus)Hasbullah El-fajarОценок пока нет
- Neraca Massa Energi Reaktor SlakerДокумент9 страницNeraca Massa Energi Reaktor Slakeroshin sinar hati siahaanОценок пока нет
- Sample Problem #17Документ10 страницSample Problem #17Dozdi100% (10)
- Tugas Atk 1: Problem Himmelblau Edisi 7: Halaman 56Документ15 страницTugas Atk 1: Problem Himmelblau Edisi 7: Halaman 56SafefireОценок пока нет
- CHE656 2010 Homework2 SolutionsДокумент20 страницCHE656 2010 Homework2 Solutionsdinesh1989novemberОценок пока нет
- Lecture 9Документ4 страницыLecture 9Asif AliОценок пока нет
- Density of MeOH - Chemical Engineers Handbook, Perry Vol 1Документ1 страницаDensity of MeOH - Chemical Engineers Handbook, Perry Vol 1Jia Yuan ChngОценок пока нет
- Lampiran B (Neraca Panas)Документ30 страницLampiran B (Neraca Panas)Sandra IvanaОценок пока нет
- Tabel Antoine 1Документ3 страницыTabel Antoine 1atikaindrnОценок пока нет
- Heat of Solutions and SolubilityДокумент25 страницHeat of Solutions and SolubilityDanielОценок пока нет
- Chemical Reaction Engineering Chapter 6Документ133 страницыChemical Reaction Engineering Chapter 6sc9112008100% (1)
- Kelompok 4 TRK 2Документ5 страницKelompok 4 TRK 2Katharina AjengОценок пока нет
- PDF (All-In-One) MK Perancangan Pabrik Kimia (Copyright - R.Turton and J. Shaeiwitz 2012) PDFДокумент564 страницыPDF (All-In-One) MK Perancangan Pabrik Kimia (Copyright - R.Turton and J. Shaeiwitz 2012) PDFSaputra Purnawan0% (1)
- Batch Manufacture of Propylene GlycolДокумент6 страницBatch Manufacture of Propylene Glycolprassna_kamat1573Оценок пока нет
- Diagram Alir Allyl ChlorideДокумент2 страницыDiagram Alir Allyl ChlorideCeevz Batara GuruОценок пока нет
- MSDS DowthermДокумент4 страницыMSDS DowthermfebriantabbyОценок пока нет
- 6611S1TKCE60132018 - Operasi Teknik Kimia III - Pertemuan 6 - Materi TambahanДокумент32 страницы6611S1TKCE60132018 - Operasi Teknik Kimia III - Pertemuan 6 - Materi TambahanDody FirmansyahОценок пока нет
- Neraca Energi Reaktor Word Kelompok 14Документ8 страницNeraca Energi Reaktor Word Kelompok 14Rani khairaniОценок пока нет
- Sizing StrippingДокумент14 страницSizing StrippingEka trisnawatiОценок пока нет
- Bioprocess Engineering Principles (Second Edition) - Chapter 12Документ105 страницBioprocess Engineering Principles (Second Edition) - Chapter 12Jhoan SierraОценок пока нет
- Data Konstanta AntoineДокумент32 страницыData Konstanta AntoineSurya NingrumОценок пока нет
- Presentation Lumped Parameter Model, Conservation of Mass, Reaction (Group 6) FixДокумент16 страницPresentation Lumped Parameter Model, Conservation of Mass, Reaction (Group 6) FixPrayogo KuntoroОценок пока нет
- Tugas TRK Ii CH 11Документ10 страницTugas TRK Ii CH 11Samuel Bagas Wahyu Santoso100% (2)
- Rangkuman Rumus TRKДокумент1 страницаRangkuman Rumus TRKNabilaFatinKamilasariОценок пока нет
- Tugas 1 Termo 2 - Rabu - Putra Maulana - 5213415062Документ8 страницTugas 1 Termo 2 - Rabu - Putra Maulana - 5213415062Putra MaulanaОценок пока нет
- Reaktor Trickle BedДокумент8 страницReaktor Trickle BedAgaОценок пока нет
- Liquid-Liquid Extraction OTKДокумент38 страницLiquid-Liquid Extraction OTKJaffarudin Janu WahyudiОценок пока нет
- (Solved) 8.4-3. Effect of Evaporator Pressure On Capacity and Product..Документ3 страницы(Solved) 8.4-3. Effect of Evaporator Pressure On Capacity and Product..CycuОценок пока нет
- Chapter 3 LevenspielДокумент40 страницChapter 3 LevenspielJohn Patrick DagleОценок пока нет
- NM Doni BaruДокумент47 страницNM Doni BaruAhmad SupraptoОценок пока нет
- Latihan Soal Otk 1Документ3 страницыLatihan Soal Otk 1Sintan TiaraОценок пока нет
- Chương 1 - Bài TậpДокумент25 страницChương 1 - Bài TậpTÍN Phạm Nguyễn TrọngОценок пока нет
- Che122xrev1 PDFДокумент4 страницыChe122xrev1 PDFSharlene Kim100% (1)
- Tug Asmik Ro Biolog Ike Lomp OkДокумент9 страницTug Asmik Ro Biolog Ike Lomp OkRetta EmeldaОценок пока нет
- Rahmanda Luthfia - Tugas 1Документ9 страницRahmanda Luthfia - Tugas 1Rahmanda LuthfiaОценок пока нет
- Tutorial SolutionsДокумент26 страницTutorial SolutionsshubhamОценок пока нет
- Heat Capacities of Liquids and Gases PDFДокумент4 страницыHeat Capacities of Liquids and Gases PDFAminEsmaeili100% (1)
- Thermo1 AssignentДокумент6 страницThermo1 AssignentmasmashitahОценок пока нет
- Ayırma İşlemleri SorularДокумент9 страницAyırma İşlemleri SorularElif Yaren Öztürk0% (1)
- Tugas2 ParalelB 4Документ14 страницTugas2 ParalelB 4Thobroni AkbarОценок пока нет
- Assignment 1 TRK Dila FirizqinaДокумент2 страницыAssignment 1 TRK Dila FirizqinaDilaFirizqinaОценок пока нет
- Exercise Chapter 5Документ8 страницExercise Chapter 5Arics ChiengОценок пока нет
- CHNG 3004 - 2019-2020 AssignmentsДокумент26 страницCHNG 3004 - 2019-2020 AssignmentsXheikhKaleem100% (1)
- Interpretation of Data From Reactor Batch IДокумент7 страницInterpretation of Data From Reactor Batch IXxxОценок пока нет
- Kinetic Models For Nonelementary Reactions: Non Chain ReactionДокумент6 страницKinetic Models For Nonelementary Reactions: Non Chain ReactionXxxОценок пока нет
- 6.1. Principles of Size ReductionДокумент2 страницы6.1. Principles of Size ReductionXxxОценок пока нет
- Algoritm of Solution Chem. Reaction Eng. (Intepretation of Batch Data) BatchДокумент2 страницыAlgoritm of Solution Chem. Reaction Eng. (Intepretation of Batch Data) BatchXxxОценок пока нет
- Clairaut's TheoremДокумент3 страницыClairaut's TheoremDeep JoshiОценок пока нет
- Thermal Stress Analysis of Rectangular Plate Due To Convection Using Finite Element MethodДокумент7 страницThermal Stress Analysis of Rectangular Plate Due To Convection Using Finite Element Methodزهرة اللوتسОценок пока нет
- CE306 Shallow and Deep FoundationsДокумент34 страницыCE306 Shallow and Deep FoundationsMustafa LuayОценок пока нет
- Fem Analysis of Jack-Up Spudcane Penetration PDFДокумент10 страницFem Analysis of Jack-Up Spudcane Penetration PDFSơn Nguyễn-LêОценок пока нет
- Micro Nutrients & Macro NutrientsДокумент18 страницMicro Nutrients & Macro Nutrientssde100% (1)
- 18 Quantitative Aspects of Chemical ChangeДокумент30 страниц18 Quantitative Aspects of Chemical Changeapi-235269401Оценок пока нет
- Hydrogen Flake BehavioursДокумент12 страницHydrogen Flake BehavioursAlex AjuОценок пока нет
- Ab-Initio Simulations of Materials Using VASP PDFДокумент35 страницAb-Initio Simulations of Materials Using VASP PDFjie shiОценок пока нет
- GATE-PYQ-Topicwise Noor Ul Huda SirДокумент585 страницGATE-PYQ-Topicwise Noor Ul Huda SirSudha balakrishnan100% (2)
- Radiochemistry of Rhodium.Документ83 страницыRadiochemistry of Rhodium.Richard.nlОценок пока нет
- Engineeringexperv 00000 I 00146Документ60 страницEngineeringexperv 00000 I 00146LALEОценок пока нет
- LunaGrid Considerations For Developing An Expandable & Distributed Lunar Power GridДокумент1 страницаLunaGrid Considerations For Developing An Expandable & Distributed Lunar Power Gridewillia13Оценок пока нет
- Use of Nanomaterials in Coatings 0 PDFДокумент18 страницUse of Nanomaterials in Coatings 0 PDFCong ChinhОценок пока нет
- Perten Falling Number Method ChecklistДокумент2 страницыPerten Falling Number Method ChecklistOSMANОценок пока нет
- Die AttachДокумент8 страницDie AttachEllen Kay Cacatian100% (1)
- M1 Lesson 1 History: The Field of Dental Materials Has Undergone More of A Revolution Than AnДокумент69 страницM1 Lesson 1 History: The Field of Dental Materials Has Undergone More of A Revolution Than AnMarian AusanОценок пока нет
- Thermal InsulationДокумент28 страницThermal Insulationferozbabu100% (2)
- Evaporation Chemical Engineering Series 2 PDF FreeДокумент52 страницыEvaporation Chemical Engineering Series 2 PDF FreeBagus Dina AkadahОценок пока нет
- Chemistry IA Example 2Документ12 страницChemistry IA Example 2Vanessa Tumanggor100% (1)
- CHEM 331 Kraus Ihazlett 1 Chapter10Документ10 страницCHEM 331 Kraus Ihazlett 1 Chapter10Ahmed Sideeg100% (1)
- ReviewerДокумент8 страницReviewermaylynXiXОценок пока нет
- Weekly Learning PlanДокумент5 страницWeekly Learning PlanRjane CañeteОценок пока нет
- Fluid Flow Through Packed BedДокумент9 страницFluid Flow Through Packed BedAmartya MitraОценок пока нет
- Corrosion Application Library ManualДокумент230 страницCorrosion Application Library ManualMiguel Angel Holguin MontañoОценок пока нет
- Principles of DistillationДокумент30 страницPrinciples of DistillationAhmed Mohamed KhalilОценок пока нет
- Factors Affecting Enzyme-Catalyzed Reactions: November 2012Документ32 страницыFactors Affecting Enzyme-Catalyzed Reactions: November 2012trang ngôОценок пока нет
- Lecture 5 - Aldehydes & KetonesДокумент93 страницыLecture 5 - Aldehydes & KetonesQutaiba Ibrahim100% (1)
- TDS-Structuro-504-Saudi-Arabia-A High Performance Concrete HyperplasticiserДокумент2 страницыTDS-Structuro-504-Saudi-Arabia-A High Performance Concrete HyperplasticiserSHAIK ASIMUDDINОценок пока нет
- Thesis Experimental Research Lemon: Source of EnergyДокумент4 страницыThesis Experimental Research Lemon: Source of Energyfranz anthonyОценок пока нет
- Chapter 13 Assertion-Reason QuestionsДокумент3 страницыChapter 13 Assertion-Reason Questionsteresa tsoiОценок пока нет