Академический Документы
Профессиональный Документы
Культура Документы
Earth Battery
Загружено:
Bujar DalipiАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Earth Battery
Загружено:
Bujar DalipiАвторское право:
Доступные форматы
See discussions, stats, and author profiles for this publication at: https://www.researchgate.
net/publication/4347039
Experimental study of earth batteries
Conference Paper · April 2008
DOI: 10.1109/ICEE.2008.4553917 · Source: IEEE Xplore
CITATIONS READS
3 15,198
3 authors:
Nasrullah Khan Zahid Saleem
115 PUBLICATIONS 863 CITATIONS
Abasyn University
28 PUBLICATIONS 164 CITATIONS
SEE PROFILE
SEE PROFILE
Naeem Abas Kalair
University of Gujrat
74 PUBLICATIONS 682 CITATIONS
SEE PROFILE
Some of the authors of this publication are also working on these related projects:
Smart Solar Refrigeration System with Flat Plate Collector. View project
Laser Testing View project
All content following this page was uploaded by Naeem Abas Kalair on 25 March 2017.
The user has requested enhancement of the downloaded file.
Second International Conference on Electrical Engineering
25-26 March 2008
University of Engineering and Technology, Lahore (Pakistan)
Experimental Study of Earth Batteries
N. Khan, Z. Saleem, N. Abas*
COMSATS Institute of Information Technology, H-8/1, Johr Campus Islamabad, Pakistan
*Department of Electrical Engineering, University of Gujrat, Gujrat, Pakistan.
nasrullahk@yahoo.com
Abstract: We report successful design, construction and operation of Although, uranium [14] and coal [9] would continue to exist
an earth battery as an alternate energy source for low power electric for few centuries but they can not replace oil and gas despite
supply applications. Different combinations of metallic and non- risks of radioactivity hazards (plutonium) and greenhouse
metallic solid, liquid and gas electrodes were investigated for gases (CO2). Either, we can stop global warming at risk of
maximum potential difference. In view of robust and cost effective
use of this natural power technology by unskilled village consumers
nuclear radiation or make the planet nuclear free at risk of
most suitable combinations of the commonly available metals were global warming due to increasing temperatures from1.4 to
selected for further detailed characteristic studies. Combinations of 5.8ºC from 1990 to 2100 by exponentially rising CO2
Magnesium anode and Coke cathode; Zinc anode and Graphite concentrations. Rise in earth surface temperature in past ten
cathode; Aluminum anode and Carbon cathode; Zinc anode and hot years (1997-2007) was about 0.6ºC. Maximum
Copper cathodes gave 2.05, 1.40, 1.10 and 0.9 volts per cell. Typical temperature has been recorded to be 52ºC in major cities of
rated power of a single Zn-Cu cell was measured to be few tens of Pakistan and 46ºC in Greece. Cool the home and heat the
microamperes. Small power electronic devices such as calculators, planet or adapt to natural ways of life. We must stop use of
electronic watches, baby toys and cell phones and white light LEDs excessive energy for entertainment and retune ourselves to
were operated on site. The voltage level was found to increase
linearly by connecting multiple earth battery cells in series like
new lifestyles requiring minimum amount of energy in the
commercial lead acid battery. The load current was found to form of cooling or heating. The scientists must work hard to
increase by connecting earth cells in parallel. The source current explore new sources of energy else be prepared to be
capacities were also found to increase by increasing surface areas of perished soon in a great energy war or global greenhouse
the electrodes. However, single cell voltage was found to remain effect none knows which prevails earlier. This work is a
constant irrespective of the electrode sizes. This paper reports very honest effort to investigate the possibility of using
detailed characteristic study of the most cost effective and accessible earth batteries for remote village lighting, communication
metal electrodes earth batteries. Operation of earth battery as a free signaling and driving small scale electronic loads where
electricity source was demonstrated successfully. there is no alternate source of electricity or simple to
conserve electricity. Assuming uniform electrode profile the
potentials of some common metals electrode pairs in soils
I. INTRODUCTION are shown in Table 1. [15-17].
Reported free energy holy grails may include electrostatic Table 1 Potential of Common Metals Suitable for Earth Battery
motors, geo-magnetic generators [1-2], air [3], sea [4] and Anode materials Cathode materials Battery
earth batteries [5-8]. Some free energy believers have often Material E (V) Material E (V) Volts
been focusing on the perpetual motion machines using magnesium -1.75 coke +0.30 2.05
scientifically unworkable ideas such as over unity devices, zinc -1.10 graphite +0.30 1.40
zinc -1.10 copper +0.20 0.90
millennium motors, resonance based self-charging and free aluminium -0.80 carbon +0.30 1.10
wheeling devices. There exists nothing as free energy source iron -0.50 coal +0.30 0.80
such as mutual powering motor-generator set without any net
input or gravity based free running machines or negative To test the possibility of higher currents and voltage a few
resistance based amplification. However, earth soil chemical large size C, Mg and Al electrodes are under construction or
reactions and electron affinity based earth batteries may be testing. Unlike air batteries used in vehicles the earth
explored for low to high voltage DC potential to drive small batteries have very low Wh capacities. It can not drive even
scale white emission LED lighting loads in remote hilly areas ordinary motorized baby toys. Above earth battery failed to
or small scale electronic devices. They can also be considered drive even an LED despite 0.7mA current due to low
to replace high voltage low current charging power supplies voltage. It had an average 0.63 W power. It was still too
or ionization power supplies. Like earth batteries the sea small to drive any motorized load except electronic digital
batteries also may be considered for similar applications. clock. A simple air battery may consist of aluminum foil (or
However, air batteries can be used for bulk power production magnesium) and activated charcoal (or iron). Oxygen from
and grid system operation [3]. In view of global energy crisis air may penetrate through saltwater soaked paper to react
to be caused by natural end of oil and gas within next 50 to with aluminum. Electrodes attached to aluminum and
60 years time [9-11], it has become very important to look for carbon may produce enough useful voltage. Air battery cell
alternative energy sources to hold back the human race from voltage depends upon reduction potential. Typical reduction
engagement to a great energy war [12-13].
978-1-4244-2293-7/08/$25.00 ©2008 IEEE.
potentials of various materials at STP are shown in Table 2
[16-19]
Table 2 Standard Reduction Potentials of Elements at 25ºC
Fig.1 Copper (south)-Zinc (north) earth battery voltages and
Per cell voltage ranges of air batteries are much lower than currents
air batteries. The best, Mg-C, earth battery has a maximum
2.05 volts whilst the best Li+(aq)-F2(g) air battery has 5.915 When the same experiment was repeated inside lab using
volts. However, air battery design needs to consider several insulated box mud cells the voltage and current was found
other economic aspects for commercial use. A common Zn- quite stable. It was supposed that the measurements made
Air battery can supply 312kWh in comparison with 22kWh outside on bare earth might have extra telluric earth currents
NiCd battery. Together they can power 200HP traction drive in addition to the normal earth battery currents. Further to
at speed of 20mph or 35 mph using higher capacity batteries. estimate the impact of telluric earth currents on the natural
Recent trends are focused on increasing speed to 55mph to directions on measured values of currents and voltages, the
cope with energy crisis. This technology is in use in several zinc electrode was fixed in earth and copper electrode was
countries since years. Air battery energy to weight and energy rotated for multiple directions from 0º (north) to 90º (east)
to size ratios are 200-250Wh/kg and 300-375 Wh/L. to180º (south) and to 270º (west). The voltages and currents
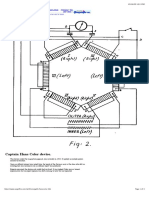
at fixed radius of 9 feet circle were found to vary slightly
vary in magnitudes as shown in Fig.2.
II. EXPERIMENTAL SETUP
An experimental study was conducted to measure exact
voltages and currents of an earth battery cells consisting of
zinc and copper electrodes. The electrodes arrangement on
earth’s surface in open air environment consists of simple
pricking of pointed electrodes on earth’s surface. The
electrode soil reaction voltage 0.92V may be used to drive
small scale lighting and electronic loads. Outside on bare
earth the currents and voltages were found higher at smaller
distances and lower at relatively larger distances between
cathode and anodes. The voltages and currents readings were
found unstable on the digital multi-meter. Repetition of above
experiment with interchange of electrodes from north to south
resulted in relatively increased voltages and currents.
Average magnitudes of voltages and currents were measured
to be 0.91±0.15V and 0.7±0.25mA for multiple electrodes.
Fig.2 Earth battery V/I characteristics for fixed zinc and
Earth battery potential depends upon the electrode materials
mobile copper electrode.
and their standard reduction potentials. If we choose higher
positive and negative reduction materials the earth battery
Electrode did not chemically corrode even after 8 to 9 hrs
voltage can be enhanced. Theoretical voltage of Zn-Cu earth
continuous use. Stronger currents flow from south to north
battery is 0.92V but our measurements conducted with UNI-
and weaker currents from east to west. Currents were also
T professional digital VOAM # 1050444792 (Korea) were
stronger for positive north and negative south electrodes.
about 0.90±0.25V. To construct a high voltage battery
Currents were found to flow from south to north. However,
suitable electrodes must be chosen. Common metals behave
it was not possible to connect earth battery cells in series to
similarly except current magnitude depends on electrode
increase the voltage as the electrodes from bottom become
surface areas. Variation of measured fluctuating voltages and
short circuited through earth electrolyte materials.
currents are shown in Fig.1.
Nevertheless, parallel connection of cells resulted in
increased currents due to increased surface areas. Spiral
design of electrodes due to large surface areas increases the
current magnitudes. Maximum magnitude of the measured
voltage was found to be 0.9 ±0.35 volts with currents in the
range of 3±0.25 A. When the same experiment was repeated
outside on open land the magnitude of current increased to
15±10 A. Both the current and magnitudes continued to
oscillate as if the some random potential source in addition to
normal soil reaction voltage was found modulating the
constant DC earth battery voltage.
III. SERIAL OPERATION OF EARTH BATTERIES
Due to short circuiting of electrodes the voltage can not
increase on bare earth surface. We need to isolate individual
cells to add up the voltage. To demonstrate serial addition of
voltages 13 DC battery cells were prepared in separate paper
boxes. The isolated earth battery cells were connected in b. A four cells 2.43V/0.20mA earth battery
series to increase the voltage as shown in Fig. 3. The distance
between Zn and Cu electrodes in different cells varied from 8 Fig.3. Experimental demonstration of serial connection of
to 10cm in Fig .3 (a) and 0.5 to 1cm in Fig.3 (b). earth batteries
The voltage varied from 10 to 12V DC with low current but
still able to light up an LED. The mud resistance between the
electrodes was tens of M . To reduce the resistance a thin
film approach was applied by appending mud coatings on the
16 inch square I mm thick copper and zinc plates as shown in
Fig.4.
Fig.4 Four inch square Zn/Cu plate electrodes 2.5V/30 A
earth battery
Four cells connected in series produced 2.5 to 3.25V DC
with 30 A current due to larger surface areas. It is to note
the voltage and current depend upon moisture content in dry
much. When it is complete dry the current reduces to zero
due to high resistance between electrodes. The reaction of
metal with soil requires moisture. Better if we go for mud
electrolyte instead of simple dry mud. The batteries
electrodes become partially rusted after long term operation.
A few results on rusting have already been published
elsewhere [20]. Further studies on large surface areas of
multiple electrodes are under investigation now.
IV. CONCLUSIONS
Results of experimental study on earth batteries using
copper and zinc electrodes are very encouraging. The initial
results for month operation of earth batteries has shown
reasonable potential for use in remote locations for
a. A twelve cells 10.30V/45mA earth battery
signaling as well as charging cell phone and white light
illumination applications. This interesting study was
undertaken as part of HEC funded research project on [1] A. A. Fesquet, “Oliver Byrne, and John Percy,”
Renewable Energy category. Being UET graduate (1984) I The Practical Metal-worker's Assistant. H.C. Baird
feel a lot pleasure to present this research work through ICEE & Co., pp. 529-530, 1878.
2008 held in UET Lahore, Pakistan. [1] E. Katz, "Alexander Bain". The history of
electrochemistry, electricity and electronics;
ACKNOWLEDGMENTS Biosensors & Bioelectronics.
[1] R. J. Edward,” Measurement of Soil Resistivity &
This study was conducted as part of a Pakistan Higher Calculation of Earth Electrode Resistance. 15th
Education Commission funded renewable energy project no. February 1998.
20-717: Minimum Ignition Study of Combustible Fuel Gases, [1] N. Khan, N. Mariun, Z. Saleem, N. Abas, “Fossil
in the Department of Electrical Engineering, CIIT Johr Fuels, New Energy Sources, and the Great Energy
Campus Islamabad, Pakistan. Crisis,” Renewable & Sustainable Energy
Reviews, Online: December 2007.
REFERENCES
[1] Gish, O. H., “The Natural Electric Currents in the
Earth's Crust,” The Scientific Monthly, Vol. 32, pp.
5-2, 1989.
[1] G. M. Hopkins, “Experimental Science:Elementary,
Practical and Experimental Physics. Munn & Co.,.
pp. 437 – 451, 1902.
[1] J. Cooper, “Powering Future Vehicles with
Refuelable Zinc/Air Battery,” Science &
Technology Review, pp. 6-13, October 1995.
[1] Lord Kelvin, Sea Battery: Method and Apparatus,
US Pat. No. 4153757, End of 1800s.
[1] Ryeczek, "U.S. Patent 4,457,988 Earth battery".
July 3, 1984.
[1] Daniel Drawbaugh, "U.S. Patent 211,322 Earth
battery for electric clocks". 1800s.
[1] M. Emme, "U.S. Patent 495,582 Ground generator
of electricity". 1900s.
[1] Dieckmann, George F., "U.S. Patent 329,724
Electric Earth Battery". November 3, 1885.
[1] K.S. Deffeyes, “Hubert’s Peak: The Impending
World Oil Shortage,” Princeton University Press.
2002: ISBN 0-691-09086-6.
[1] D. Goodstein,” Out of Gas: The End of the Age of
Oil. W. W. Norton’ Book 2005
ISBN 0-393-05857-3.
[1] H.H. Rogner,” An Assessment of World
Hydrocarbon Resources,” Annu. Rev. Energy
Environ, Vol. 22, pp. 217-262, 1997.
[1] M.C. Ruppert,” Crossing the Rubicon: The Decline
of the American Empire at the End of the Age of
Oil,” New Society. 2005: ISBN-13: 978-
0865715400.
[1] L.C. Kleveman,” The New Great Game: Blood and
Oil in Central Asia,” Atlantic Monthly Press. 2004:
ISBN 0-87113-906-5.
[1] K.S. Deffeyes, I.D. MacGregor, “ World Uranium
Resources, Scientific America,” Vol. 242, pp. 66-67.
1980.
[1] http://en.wikipedia.org/wiki/Earth_battery
[1] James Napier, “A manual of electro-metallurgy,” pp.
48-49, 1876
View publication stats
Вам также может понравиться
- Stanley Meyer Big Bobbin Builders Guide 2019 v1: Very Rare Guide for How to Assemble Water Fueled injector tri-filar bobbinОт EverandStanley Meyer Big Bobbin Builders Guide 2019 v1: Very Rare Guide for How to Assemble Water Fueled injector tri-filar bobbinОценок пока нет
- 3 Making An Earth BatteryДокумент2 страницы3 Making An Earth Batteryouchhh88Оценок пока нет
- 1875 Patent On Earth BatteriesДокумент22 страницы1875 Patent On Earth Batteriesjoetylor100% (1)
- The TransformerДокумент21 страницаThe TransformerAshok KumarОценок пока нет
- Muammer Yildiz - Over-Unity Homopolar Electrical Generator - Patent, ArticlesДокумент29 страницMuammer Yildiz - Over-Unity Homopolar Electrical Generator - Patent, ArticlesMohd FakhriОценок пока нет
- The Jospeh H.Cater Free Energy Device: WWW - Artmemory.co - Jp/crystalДокумент20 страницThe Jospeh H.Cater Free Energy Device: WWW - Artmemory.co - Jp/crystalBSNОценок пока нет
- MEG Patent PDFДокумент19 страницMEG Patent PDFallhigorОценок пока нет
- Capacitors That Recharge Themselves PDFДокумент10 страницCapacitors That Recharge Themselves PDFivoОценок пока нет
- Earth BatteryДокумент4 страницыEarth BatteryTariq PatelОценок пока нет
- Moray Cosmic EnergyДокумент8 страницMoray Cosmic EnergyDwi Sri SudartiОценок пока нет
- ERR Power Station Project BookletДокумент18 страницERR Power Station Project BookletAnonymous C0KBah6TEqОценок пока нет
- The Complete Free Energy Bearden-MEG Study PackДокумент3 страницыThe Complete Free Energy Bearden-MEG Study PackWalterSobchakОценок пока нет
- Magnetron Water Powered Lawnmower Engine PlansДокумент2 страницыMagnetron Water Powered Lawnmower Engine Plansshawnleegabriel100% (3)
- CHEMALLOY - A New Alloy For The Science StudentДокумент2 страницыCHEMALLOY - A New Alloy For The Science StudentPeter Benedikt WeberОценок пока нет
- Capacitors That Recharge ThemselvesДокумент10 страницCapacitors That Recharge Themselvesdakkid100% (1)
- Hybrid - Magrav - Generator - v1 RomanaДокумент2 страницыHybrid - Magrav - Generator - v1 RomanaLUCIAN HOMONE100% (1)
- Uses of Sound EnergyДокумент1 страницаUses of Sound Energyxylaxander100% (1)
- Tesla Free Energy CollectorДокумент7 страницTesla Free Energy CollectorJelle VanherckОценок пока нет
- The Resonance Energy Device Explained: PrefaceДокумент39 страницThe Resonance Energy Device Explained: PrefaceR. K GuptaОценок пока нет
- Making A Self-Powered GeneratorДокумент11 страницMaking A Self-Powered GeneratorAwwad IyadОценок пока нет
- Energy AccumulatorДокумент2 страницыEnergy Accumulator47jarvОценок пока нет
- Bedini SG Intermediate HandbookДокумент8 страницBedini SG Intermediate HandbookVlad Adrian0% (1)
- 1 Modern Radiant Energy Circuit PDFДокумент3 страницы1 Modern Radiant Energy Circuit PDFavisenicОценок пока нет
- Transmutation of Elements Toby Grotz: Thomas Henry Moray and The Transmutation of ElementsДокумент3 страницыTransmutation of Elements Toby Grotz: Thomas Henry Moray and The Transmutation of Elementsstella888100% (1)
- Transformers PDFДокумент6 страницTransformers PDFVasileSpireaОценок пока нет
- Panacea-BOCAF On-Line UniversityДокумент39 страницPanacea-BOCAF On-Line UniversityMihai DanielОценок пока нет
- Simple Free-Energy Devices: Chapter 33: The Simplified Perpetual LightДокумент4 страницыSimple Free-Energy Devices: Chapter 33: The Simplified Perpetual LightpranalarОценок пока нет
- Plasma Generator v2 With PhotoДокумент4 страницыPlasma Generator v2 With PhotoRodolfo JarquinОценок пока нет
- A Perpetual LightДокумент7 страницA Perpetual Lightsuherlan endanОценок пока нет
- Bearden - Tech Papers - Fogal Transistor Notes and ReferenceДокумент29 страницBearden - Tech Papers - Fogal Transistor Notes and Referencelightingfastno808Оценок пока нет
- OSE Free Energy PendulumДокумент45 страницOSE Free Energy PendulumHans Wahler0% (1)
- Advanced Energy and Propulsion Systems Based On Chronal Reaction Method - Alexander V. Frolov PDFДокумент5 страницAdvanced Energy and Propulsion Systems Based On Chronal Reaction Method - Alexander V. Frolov PDFGherghe BogdanОценок пока нет
- Nerator 1 0 7 D PDFДокумент45 страницNerator 1 0 7 D PDFAnonymous u5128WZ1KОценок пока нет
- Goldenseal: An Annotated BibliographyДокумент76 страницGoldenseal: An Annotated BibliographyMarisela Sanchez Ch100% (1)
- Agha Waqar's Water KitДокумент19 страницAgha Waqar's Water Kitfas133100% (1)
- Part 3 Zpe PDFДокумент11 страницPart 3 Zpe PDFAnonymous u5128WZ1KОценок пока нет
- Tesla Switch Solar Charger To Debut at Bedini ConferenceДокумент8 страницTesla Switch Solar Charger To Debut at Bedini Conferenceutorrent411Оценок пока нет
- Prentice Earth Energy TapДокумент7 страницPrentice Earth Energy TapGeorggeОценок пока нет
- The Inventions of CaptainДокумент3 страницыThe Inventions of CaptainAngela0220Оценок пока нет
- Hendershot MRXДокумент16 страницHendershot MRXvictor munteanОценок пока нет
- Nicola Tesla Electroradiant EffectДокумент16 страницNicola Tesla Electroradiant Effectrodolfo barbosaОценок пока нет
- Moray SecretДокумент13 страницMoray SecretOvidiu FratuОценок пока нет
- QEG Parts WorksheetДокумент4 страницыQEG Parts Worksheetrockafella09Оценок пока нет
- Earth Batteries PDFДокумент0 страницEarth Batteries PDFMarìa Angélica Guerreiro0% (1)
- Motionless Electromagnetic GeneratorДокумент9 страницMotionless Electromagnetic GeneratorViswa TejaОценок пока нет
- Tesla Coil ImpedanceДокумент16 страницTesla Coil ImpedanceMagnethos100% (1)
- Teslas Electric Car No2Документ5 страницTeslas Electric Car No2bman0051401Оценок пока нет
- The Hubbard Energy Transformer by Gaston Burridge, Fate Magazine 1956Документ24 страницыThe Hubbard Energy Transformer by Gaston Burridge, Fate Magazine 1956pplowe2305tedОценок пока нет
- HTTP://WWW - Ebay.com/sch/i.html? From R40& Sacat 0& NKW Magnets& PGN 5& SKC 200&rt NCДокумент36 страницHTTP://WWW - Ebay.com/sch/i.html? From R40& Sacat 0& NKW Magnets& PGN 5& SKC 200&rt NCZoran AleksicОценок пока нет
- Flyback Driver CircuitoДокумент15 страницFlyback Driver CircuitoCarlos Sarmiento LoraОценок пока нет
- Cold Electricity Circuit Diagram With Capacitors - by UFOpolitics - Tír Na Saor Land of The FreeДокумент6 страницCold Electricity Circuit Diagram With Capacitors - by UFOpolitics - Tír Na Saor Land of The FreeSadegh Simorgh100% (4)
- Steven Marks Tpu Rev1Документ63 страницыSteven Marks Tpu Rev1catedralwebОценок пока нет
- Adams Motor DetaljerДокумент18 страницAdams Motor DetaljerGeorg LidtveitОценок пока нет
- Bahan Mentah PKMДокумент11 страницBahan Mentah PKMRizky AzizahОценок пока нет
- J Mater Chem A201647207-7213Документ8 страницJ Mater Chem A201647207-7213alvinjhun alejoОценок пока нет
- Book Review Power Souces For Electric VehiclesДокумент2 страницыBook Review Power Souces For Electric VehiclesLUCIANA DE SOUZA FREIREОценок пока нет
- Air Cathodes 05420955Документ6 страницAir Cathodes 05420955Dean JenningsОценок пока нет
- Solid Oxide Electrolyzer Cell Modeling: A Review: Journal Homepage:papers - Itc.pw - Edu.plДокумент31 страницаSolid Oxide Electrolyzer Cell Modeling: A Review: Journal Homepage:papers - Itc.pw - Edu.pl1212Оценок пока нет
- Fuel Cells PaperДокумент20 страницFuel Cells PaperBig FruitОценок пока нет
- Fuel Cells: A Promising Tecnology For Distributed Power GenerationДокумент10 страницFuel Cells: A Promising Tecnology For Distributed Power GenerationDrYogesh DhoteОценок пока нет
- Mono Module: Pik 450MДокумент2 страницыMono Module: Pik 450MBujar DalipiОценок пока нет
- 340P PDFДокумент2 страницы340P PDFBujar DalipiОценок пока нет
- Pik High Quality PV Modules SeriesДокумент2 страницыPik High Quality PV Modules SeriesBujar DalipiОценок пока нет
- Wiring Diagram For Photonic Universe KIT3K-1440-7KWHДокумент1 страницаWiring Diagram For Photonic Universe KIT3K-1440-7KWHBujar DalipiОценок пока нет
- Polycrystalline PV ModulesДокумент2 страницыPolycrystalline PV ModulesBujar DalipiОценок пока нет
- Hyrje Ne Programim Ne PHP-LeksionДокумент8 страницHyrje Ne Programim Ne PHP-LeksionBujar DalipiОценок пока нет
- Nuclear EmergencyДокумент4 страницыNuclear EmergencyBujar DalipiОценок пока нет
- Sag DB en PDFДокумент2 страницыSag DB en PDFMiguel Angel Pacahuala CristobalОценок пока нет
- Basic Layout of OLTДокумент3 страницыBasic Layout of OLTgulasbotОценок пока нет
- Antennas and Radar For Environmental Scientists and EngineersДокумент400 страницAntennas and Radar For Environmental Scientists and EngineersNabil Dakhli100% (1)
- Chap4 Student VersionДокумент39 страницChap4 Student VersionAzrif MoskamОценок пока нет
- Digital System Design - 0Документ15 страницDigital System Design - 0Đoàn Vũ Phú VinhОценок пока нет
- Books in The Ieee Press Series On Power EngineeringДокумент490 страницBooks in The Ieee Press Series On Power EngineeringFranklin CutocaОценок пока нет
- Design Software 1Документ2 страницыDesign Software 1anbarasuval84Оценок пока нет
- Rexroth IndraMotion For Handling IndraLogic L20 - L40. Application Manual DOK-IM - HA - IL - APPL - PR02-EN-DДокумент79 страницRexroth IndraMotion For Handling IndraLogic L20 - L40. Application Manual DOK-IM - HA - IL - APPL - PR02-EN-DRobinsonDanielDosSantosОценок пока нет
- Ventilador Bear 1000 Especificaciones TecnicasДокумент2 страницыVentilador Bear 1000 Especificaciones TecnicasDaniel F. Guerrero P.Оценок пока нет
- Calibration of Linear Displacement Sensor Systems Used To Measure MicromotionДокумент4 страницыCalibration of Linear Displacement Sensor Systems Used To Measure MicromotionAhmad Zubair RasulyОценок пока нет
- Lecture#7Документ43 страницыLecture#720pwmct0739Оценок пока нет
- PIN120 (English Manual)Документ12 страницPIN120 (English Manual)aldokecoОценок пока нет
- Barras 2019Документ17 страницBarras 2019Reginaldo SilvaОценок пока нет
- Bugreport Ysl PKQ1.181203.001 2020 01 04 19 37 44 Dumpstate - Log 20606Документ18 страницBugreport Ysl PKQ1.181203.001 2020 01 04 19 37 44 Dumpstate - Log 20606Sandeep TaleОценок пока нет
- Feynman Path-Integral: Andreas ToppДокумент80 страницFeynman Path-Integral: Andreas ToppVigneshwaran KannanОценок пока нет
- Data Sheet: para Light Electronics Co., LTDДокумент14 страницData Sheet: para Light Electronics Co., LTDmuaadhОценок пока нет
- Susol - VCB - E - 1303 (07-11-2013)Документ152 страницыSusol - VCB - E - 1303 (07-11-2013)lymacsausarangОценок пока нет
- DIAL's RequirementsДокумент57 страницDIAL's RequirementsShaikh Saeed AlamОценок пока нет
- 171001Документ2 страницы171001vishalsanziraОценок пока нет
- AdrenaLinn Sync User GuideДокумент1 страницаAdrenaLinn Sync User GuidesОценок пока нет
- MV Switchgear Circuit Breaker Inspection and Test Procedure: October 2019Документ5 страницMV Switchgear Circuit Breaker Inspection and Test Procedure: October 2019noman ahmadОценок пока нет
- M-550 Power Service Manual: Model No: 3399 Drawring No: Customer: Model No: M-550 Power Rev, DateДокумент65 страницM-550 Power Service Manual: Model No: 3399 Drawring No: Customer: Model No: M-550 Power Rev, Datealbinopu2liuОценок пока нет
- Examples of MPIДокумент3 страницыExamples of MPIzaw lin ooОценок пока нет
- Final Day PresentationДокумент43 страницыFinal Day Presentationswanichatterjee0Оценок пока нет
- Insurance Premium Option: Calculation SheetДокумент44 страницыInsurance Premium Option: Calculation SheetroyalvirenОценок пока нет
- Icp-Oes Plasma Quant Pq9000Документ16 страницIcp-Oes Plasma Quant Pq9000Mari Sherlin Salisi-ChuaОценок пока нет
- Overcurrent Protective Device Coordination StudyДокумент3 страницыOvercurrent Protective Device Coordination StudySankalp TiwariОценок пока нет
- Qualcomm Client Interview Question - Downlaod VLSI FOR ALLДокумент18 страницQualcomm Client Interview Question - Downlaod VLSI FOR ALLPrajwal SОценок пока нет
- Title: Dual Trace Oscilloscope: U5 ST7735s TFT ScreenДокумент1 страницаTitle: Dual Trace Oscilloscope: U5 ST7735s TFT ScreenAbdelkader Mechernene100% (2)
- TCM1.0E TCM2.0E: May, 2008Документ69 страницTCM1.0E TCM2.0E: May, 2008JejeОценок пока нет
- Power of 10: The Once-A-Week Slow Motion Fitness RevolutionОт EverandPower of 10: The Once-A-Week Slow Motion Fitness RevolutionРейтинг: 3.5 из 5 звезд3.5/5 (11)
- Peak: The New Science of Athletic Performance That is Revolutionizing SportsОт EverandPeak: The New Science of Athletic Performance That is Revolutionizing SportsРейтинг: 5 из 5 звезд5/5 (97)
- Chair Yoga: Sit, Stretch, and Strengthen Your Way to a Happier, Healthier YouОт EverandChair Yoga: Sit, Stretch, and Strengthen Your Way to a Happier, Healthier YouРейтинг: 3.5 из 5 звезд3.5/5 (5)
- Aging Backwards: Reverse the Aging Process and Look 10 Years Younger in 30 Minutes a DayОт EverandAging Backwards: Reverse the Aging Process and Look 10 Years Younger in 30 Minutes a DayОценок пока нет
- Strength Training Over 40: The Only Weight Training Workout Book You Will Need to Maintain or Build Your Strength, Muscle Mass, Energy, Overall Fitness and Stay Healthy Without Living in the GymОт EverandStrength Training Over 40: The Only Weight Training Workout Book You Will Need to Maintain or Build Your Strength, Muscle Mass, Energy, Overall Fitness and Stay Healthy Without Living in the GymРейтинг: 4 из 5 звезд4/5 (6)
- Functional Training and Beyond: Building the Ultimate Superfunctional Body and MindОт EverandFunctional Training and Beyond: Building the Ultimate Superfunctional Body and MindРейтинг: 4.5 из 5 звезд4.5/5 (1)
- The Total Kettlebell Workout: Trade Secrets of a Personal TrainerОт EverandThe Total Kettlebell Workout: Trade Secrets of a Personal TrainerРейтинг: 5 из 5 звезд5/5 (1)
- Muscle for Life: Get Lean, Strong, and Healthy at Any Age!От EverandMuscle for Life: Get Lean, Strong, and Healthy at Any Age!Рейтинг: 4.5 из 5 звезд4.5/5 (22)
- Boundless: Upgrade Your Brain, Optimize Your Body & Defy AgingОт EverandBoundless: Upgrade Your Brain, Optimize Your Body & Defy AgingРейтинг: 4.5 из 5 звезд4.5/5 (67)
- Strong Is the New Beautiful: Embrace Your Natural Beauty, Eat Clean, and Harness Your PowerОт EverandStrong Is the New Beautiful: Embrace Your Natural Beauty, Eat Clean, and Harness Your PowerРейтинг: 4 из 5 звезд4/5 (5)
- The Yogi Code: Seven Universal Laws of Infinite SuccessОт EverandThe Yogi Code: Seven Universal Laws of Infinite SuccessРейтинг: 4.5 из 5 звезд4.5/5 (104)
- Mind Your Body: 4 Weeks to a Leaner, Healthier LifeОт EverandMind Your Body: 4 Weeks to a Leaner, Healthier LifeРейтинг: 4.5 из 5 звезд4.5/5 (5)
- The Power of Fastercise: Using the New Science of Signaling Exercise to Get Surprisingly Fit in Just a Few Minutes a DayОт EverandThe Power of Fastercise: Using the New Science of Signaling Exercise to Get Surprisingly Fit in Just a Few Minutes a DayРейтинг: 4 из 5 звезд4/5 (4)
- Endure: Mind, Body, and the Curiously Elastic Limits of Human PerformanceОт EverandEndure: Mind, Body, and the Curiously Elastic Limits of Human PerformanceРейтинг: 4.5 из 5 звезд4.5/5 (237)
- Roxane Gay & Everand Originals: Built for This: The Quiet Strength of PowerliftingОт EverandRoxane Gay & Everand Originals: Built for This: The Quiet Strength of PowerliftingРейтинг: 4.5 из 5 звезд4.5/5 (133)
- Easy Strength: How to Get a Lot Stronger Than Your Competition-And Dominate in Your SportОт EverandEasy Strength: How to Get a Lot Stronger Than Your Competition-And Dominate in Your SportРейтинг: 4.5 из 5 звезд4.5/5 (17)
- Fascial Training: With Easy Exercises To More Mobility And Less Pain (10 Minutes Fascia Workout For Home)От EverandFascial Training: With Easy Exercises To More Mobility And Less Pain (10 Minutes Fascia Workout For Home)Рейтинг: 5 из 5 звезд5/5 (3)
- Tibetan Yoga: Magical Movements of Body, Breath, and MindОт EverandTibetan Yoga: Magical Movements of Body, Breath, and MindРейтинг: 5 из 5 звезд5/5 (1)
- The Science of Getting Ripped: Proven Diet Hacks and Workout Tricks to Burn Fat and Build Muscle in Half the TimeОт EverandThe Science of Getting Ripped: Proven Diet Hacks and Workout Tricks to Burn Fat and Build Muscle in Half the TimeРейтинг: 4 из 5 звезд4/5 (8)
- Not a Diet Book: Take Control. Gain Confidence. Change Your Life.От EverandNot a Diet Book: Take Control. Gain Confidence. Change Your Life.Рейтинг: 4.5 из 5 звезд4.5/5 (124)
- Wheels of Life: A User's Guide to the Chakra SystemОт EverandWheels of Life: A User's Guide to the Chakra SystemРейтинг: 4.5 из 5 звезд4.5/5 (34)
- 80/20 Running: Run Stronger and Race Faster by Training SlowerОт Everand80/20 Running: Run Stronger and Race Faster by Training SlowerРейтинг: 4.5 из 5 звезд4.5/5 (97)
- Enter The Kettlebell!: Strength Secret of the Soviet SupermenОт EverandEnter The Kettlebell!: Strength Secret of the Soviet SupermenРейтинг: 4 из 5 звезд4/5 (29)
- True Yoga: Practicing With the Yoga Sutras for Happiness & Spiritual FulfillmentОт EverandTrue Yoga: Practicing With the Yoga Sutras for Happiness & Spiritual FulfillmentРейтинг: 4.5 из 5 звезд4.5/5 (22)
- Calisthenics: Guide for Bodyweight Exercise, Build your Dream Body in 30 MinutesОт EverandCalisthenics: Guide for Bodyweight Exercise, Build your Dream Body in 30 MinutesРейтинг: 3 из 5 звезд3/5 (5)