Академический Документы
Профессиональный Документы
Культура Документы
CRE Lab Manual
Загружено:
Nabeel BhuttaОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
CRE Lab Manual
Загружено:
Nabeel BhuttaАвторское право:
Доступные форматы
Chemical Reaction Engineering
LIST OF EQUIPMENTS
CHEMICAL REACTION ENGINEERING LABORATORY
Sr. No Equipment
1 Edibon plug flow reactor
2 Edibon batch reactor
3 Elettronica veneta CSTR
4 Elettronica veneta PFR
5 Elettronica veneta reactor
6 Elettronica veneta CSTR in series
7 Catalyzed plug flow reactor
8 Fixed bed reactor
9 Fluidized bed reactor
10 Hybrid reactor
11 Alkaline fading reaction unit
12 Hydrogenation reactor until
13 Pilot plant for sulphonation of benzene
14 Pilot plant for production of hexamethylene tetramine (HMT)
LIST OF EXPERIMENTS
NFC IET MULTAN
Chemical Reaction Engineering
Sr.No Experiment
1 To standardize a standard solution of sodium hydroxide and develop equation of
trendline.
2 Find the effect of initial molar concentration (CAo) of a key component for a
given reaction in the lab under the isothermal conditions (constant density
system) on the space time, conversion and space velocity of PFR.
3 To study the kinetics (n and k) of given reaction by using differential analysis.
4 To study the effect of volume of reaction mixture on conversion, space time and
space velocity for continuous reactor.
5 To compare the rate of given chemical reaction at room temperature with and
without agitation.
6 To find/investigate the effect of flow rate on conversion, space time and space
velocity in PFR.
7 To find the value of n and k for the given reaction by using integral analysis.
8 To study the kinetics of saponification reaction (i.e. K) at different temperatures
by using conduct metric method.
9 To study the effect of temperature on the rate of reaction in a batch reactor for a
given saponification reaction in the lab.
10 To study the effect of superficial air velocity on overall heat transfer co-efficient
for the air-steam system in a fluidized bed reactor.
11 Determine rate of conversion of hydrogen to ethane as a function of time in a
tubular flow reactor.
12 To study the effect of superficial air velocity on pressure drop in fixed and
fluidized bed conditions and compare their results.
13 To study the effect of superficial air velocity on overall heat transfer co-efficient
for the air-steam system in a hybrid reactor.
NFC IET MULTAN
Chemical Reaction Engineering
14 To determine the rate of reaction in the sulfonation of benzene on a pilot plant.
15 To plot conversion of formalin as a function of time.
16 Find out the activation energy for the hydrogenation of ethylene using magnesia-
copper oxide catalyst.
EXPERIMENT NO. 1
NFC IET MULTAN
Chemical Reaction Engineering
Object:
To standardize a standard solution of sodium hydroxide and develop equation of trend
line.
Apparatus:
Beaker, glass rod, conductivity meter, standard solution of NaOH, Cylinder.
Procedure:
1. Prepare standard solution (1 M or 0.1 M) of NaOH ( M= molarity).
2. For 1M solution of NaOH, weigh 40g of NaOH, put it in 1000ml cylinder, then add
water to make volume upto 1000ml.
3. Take 100/200ml of this standard solution in any suitable beaker and measure its
conductivity by conductivity meter.
4. Then add 10ml water each time in the same solution and measure the conductivity.
5. Take at least 10 readings.
6. For molarity of the solution each time use M1V1/n1 = M2V2/n2
7. Draw a graph between molarity (concentration) on x-axis and conductivity (mS, μS,
ppm or mg/L) on y-axis.
Observations And Calculations:
Sr. No Molarity (M) Conductivity (mS , μS, ppm)
Expected Trend (Graph):
NFC IET MULTAN
Chemical Reaction Engineering
Graph b/w conductivity and molarity
3.5
3
conductivity (mg/L, ppm)
2.5
1.5
0.5
0
0 0.5 1 1.5 2 2.5 3 3.5
Molarity ( M)
Theory:
The following topics should be covered by students
Methods of concentration measurement of a solution.
Conductivity and its units.
Calibration of conductivity meter.
Parameters which can be measured by conductivity meter.
Other techniques for the measurement of concentration of solution.
Learning Outcomes:
EXPERIMENT NO. 2
NFC IET MULTAN
Chemical Reaction Engineering
Object:
Find the effect of initial molar concentration (CAo) of a key component for a given
reaction in the lab under the isothermal conditions (constant density system) on the space time
and space velocity of PFR.
Apparatus:
Plug flow reactor
Procedure:
Following points are allowed while performing the experiment.
1. Note the initial concentration CAo of the reaction mixture.
2. Switch on the reactor and let it achieve steady state.
3. After specific time note the final concentration CAf.
4. From the values of CAo and CAf calculate XA.
5. From this XA calculate volume (V), space time (τ) and space velocity (s).
6. Plot the following graph from the calculations:
Graph between CAo and V.
Graph between CAo and τ.
Graph between CAo and s.
Observations And Calculations:
Sr.No CAo CAo CAf XA= 1- V ( liter) τ s=1/τ
(gmole/hr) (μS) (μS) (CAf/CAO) (min) (min-1)
Formulas used:
XA= 1-(CAf/CAO)
𝑋𝐴
𝑉 𝑑𝑋𝐴
=∫
𝐹𝐴𝑂 0 −𝑟𝐴
-rA = k CAo2 (1-XA)2
V/ CAoV0 = (1/(K CAo 2))(XA/(1-XA))
NFC IET MULTAN
Chemical Reaction Engineering
τ= V/V0
s = 1/τ
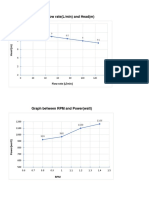
Graphs:
Graph between CAo and V.
V(L)
CA0 (gmol/L)
Graph between CAo and τ.
τ (min)
CA0 (gmol/L)
NFC IET MULTAN
Chemical Reaction Engineering
Graph between CAo and s.
s(min-1)
CA0 (gmol/L)
Theory:
The following topics should be covered by students
Concept of space time and space velocity.
Construction and working of PFR.
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 3
Object:
To study the kinetics (n and k) of given reaction by using differential analysis.
Apparatus:
Batch reactor with and without agitator, glass rod, conductivity meter, standard solution
of NaOH and ethyl acetate, Cylinder.
Reaction:
CH3COOC2H5 + NaOH → CH3COONa + C2H5Na
Procedure:
1. Take 100ml standard solution (1 M or 0.1 M) of NaOH and 100ml of ethyl acetate in
separate beakers.
2. Note the conductivity of (0.1 M) NaOH solution by conductivity meter, it will be C A0
at time t=0.
3. Put these solutions in the batch reactor simultaneously with or without agitation, as the
reaction starts, note the conductivity after every 20 seconds and continue to do so until
a constant reading is obtained.
4. Plot conductivity Vs time and apply differential analysis by plotting tangents to this
curve and then find the values of “n” and “k” after plotting a graph between ln(-dCA/dt)
vs. lnCA.
NFC IET MULTAN
Chemical Reaction Engineering
Observations And Calculations:
Sr.No Time Conductivity Points on each Slope ln(--dCA/dt) ln(CA)
(min) (ppm) tangents -dCA/dt
Graph:
CA
(mg/L)
time (t)
ln -dCA/dt
ln CA
NFC IET MULTAN
Chemical Reaction Engineering
Theory:
The following topics should be covered by students
Concept of differential analysis.
Concept of order of reaction and rate constant.
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 4
Object:
To study the effect of volume of reaction mixture on conversion, space time and space
velocity for continuous reactor.
Apparatus:
CSTR
PFR
Procedure:
1. First of all, switch on the CSTR and want till it achieves steady state. Then calculate
the initial concentration of the solution.
2. Then calculate the concentration at different intervals.
3. Then calculate the volume by collecting the solution.
4. Calculate the conversion, space time and space velocity.
5. Plot the graph between volume and conversion, volume and space time and volume and
space velocity.
6. Repeat the same procedure for PFR.
Observations And Calculations:
Sr.No CAo XA= 1- V ( liter) τ s=1/τ
(μS) (CAf/CAO) (min) (min-1)
Formulas used:
XA= 1-(CAf/CAO)
τ= V/V0
s = 1/τ
NFC IET MULTAN
Chemical Reaction Engineering
Graphs:
Graph between Vo and XA.
XA
VO (L)
Graph between Vo and τ.
τ (min)
VO (L)
Theory:
Construction and working of CSTR.
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 5
Object:
To compare the rate of given chemical reaction at room temperature with or without
agitation.
Apparatus:
Batch reactor with stirrer, beaker, conductivity meter, standard solution of NaOH and
ethyl acetate, cylinder, stop watch, stirrer.
Procedure:
1. Take 100ml or as per required standard solution (0.1 M) each for NaOH and ethyl
acetate in a beaker.
2. Put these solutions in batch reactor simultaneously.
3. First the readings without agitation at room temperature and then with agitation at room
temperature from new solutions.
4. Plot graphs between concentration vs. time and rate of chemical reaction vs. time with
and without agitation.
Observations And Calculations:
Without agitation
Using k= 6.42 L/(gmol)(min)
Sr. No Time Conductivity Conc. -rA=kCA2
(min) (ppm) (mg/L)
NFC IET MULTAN
Chemical Reaction Engineering
With agitation
Using k= 6.42 L/(gmol)(min)
Sr. No Time Conductivity Conc. -rA=kCA2
(min) (ppm) (mg/L)
Graphs:
Without
Conc. agitation
With
agitation
With
agitation
-rA
Without
agitation
NFC IET MULTAN
Chemical Reaction Engineering
Theory:
The following topics should be covered by students
Concept of rate of reaction.
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 6
Object:
To find/investigate the effect of flow rate on conversion, space time and space velocity
in PFR.
Apparatus:
Plug flow reactor
Procedure:
1. Note the initial concentration CA0 of the reaction mixture.
2. Switch on the reactor.
3. Set a specific flow rate and let the reactor achieve steady state.
4. After specific time note the final concentration CAF.
5. From the values of CA0 and CAF calculate conversion XA.
6. Also calculate space time (τ) and space velocity (s).
7. Plot the following graph from the calculations;
Graph between V0 and XA.
Graph between V0 and r.
Graph between V0 and s.
Observations And Calculations:
Observations for pump 1:
Sr.No V0 CAf XA= 1- τ s=1/τ
(ml/min) (mS) (CAf/CAO) (min) (min-1)
Observations for pump 2:
Sr.No V0 CAf XA= 1- τ s=1/τ
(ml/min) (mS) (CAf/CAO) (min) (min-1)
NFC IET MULTAN
Chemical Reaction Engineering
Formulas Used:
XA= 1-(CAf/CAO)
τ= V/V0
s = 1/τ
Graphs:
XA
VO (mL/min)
τ (min)
VO (mL/min)
NFC IET MULTAN
Chemical Reaction Engineering
s (min)-1
VO (mL/min)
Theory:
The following topics should be covered by students
Methods of concentration measurement of a solution.
Conductivity and its units.
Calibration of conductivity meter.
Parameters which can be measured by conductivity meter.
Other techniques for the measurement of concentration of solution.
Theory:
The following topics should be covered by students
Construction and working of PFR
Concept of space time and space velocity
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 7
Object:
To find the value of n and k for the given reaction by using integral analysis.
Apparatus:
Batch reactor with and without agitator, glass rod, conductivity meter, standard solution
of NaOH and ethyl acetate, cylinder.
Reaction:
CH3COOC2H5 + NaOH → CH3COONa + C2H5Na
Procedure:
1. Take 100ml standard solution (1 M or 0.1 M) of NaOH and 100ml of ethyl acetate in
separate beakers. Note the conductivity of (0.1 M) NaOH solution by conductivity
meter, it will be CA0 at time t=0.
2. Put 100ml each solution in a batch reactor in the lab simultaneously as the reaction
starts, note the conductivity (ppm,mg/L, mS, μS) by conductivity meter after every 20
sconds and prepare the following table.
3. Plot ln(CA0/CA) vs time for first order kinetics.
4. Plot (1/CA-1/ CA0) vs time with CA0=CB0 or 1/CA vs. time with “1/CA0” as an intercept;
if “CA0” is not known for 2nd order kinetics.
5. Plot ½[1/CA2-1/ CA02] vs. time for third order kinetics. If the above plots in 3,4 and 5
are perfect straight lines with more than (85-90)% of the data falling on this line,
integral analysis is correct, otherwise it fails.
6. For perfect straight line the order (n=1, 2, 3) will be known and from slope of this line
“k” can be found. Remember this method will give integral order only.
NFC IET MULTAN
Chemical Reaction Engineering
Observations And Calculations:
Time Conc. CA0/CA ln(CA0/CA) 1/CA-1/ CA0 1/CA ½[1/CA2-1/ CA02]
(min) (CA)
Theory:
The following topics should be covered by students
Concept of order and rate constant
Concept of integral analysis
Graph:
ln(CA0/CA)
Time (t)
NFC IET MULTAN
Chemical Reaction Engineering
(1/CA)
Time (t)
1/CA-1/ CA0
Time (t)
½[1/CA2-1/ CA02]
Time (t)
NFC IET MULTAN
Chemical Reaction Engineering
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 8
Object:
To study the kinetics of saponification reaction (i.e. K) at different temperatures by
using conductometric method.
Apparatus:
Batch reactor, conductivity meter, standard solution of NaOH and ethyl acetate,
cylinder, stop watch.
Procedure:
1. First take data of previous experiment i.e. between time and conductivity.
2. Then calculate C0 – Ct for all the temperatures i.e. 35 0C, 45 0C, 55 0C and 65 0C.
3. After that calculate C0 – Ct for all the temperatures.
4. Find the value of k by using the formula
k= 1/0.01(C0 – Ct/ Ct – Cf) t
5. At the end calculate kavg for all these temperatures which are our objective function.
NFC IET MULTAN
Chemical Reaction Engineering
Observations And Calculations:
At t= 35 0C
(C0 at t=0 sec/min)
(Cf at t where reading becomes constant)
Time Conductivity Conc. C0 – Ct Ct – Cf k= 1/0.01(C0 – Ct/ Ct – Cf) t
(min) mg/L or M
ppm
k1= (kavg) at 35 0C
Similar table should be prepared for temperature i.e. 35 0C, 45 0C, 55 0C and 65 0C and also
calculate the values for average “k” against each temperature i.e. k2,k3,k1.
Theory:
The following topics should be covered by students
Concept of saponification reaction.
Concept of Arrhenius law.
Knowhow of activation energy, frequency factor and rate constant.
Conductivity and its units.
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 9
Object:
To study the effect of temperature on the rate of reaction in a batch reactor for a given
saponification reaction in the lab.
Apparatus:
Beaker, conductivity meter, standard solution of NaOH and ethyl acetate, cylinder,
heating source, stop watch, stirrer.
Procedure:
1. Use the table at different temperatures from experiment no. 8.
2. Find the rate of chemical reaction against each value of time for each temperature.
3. Plot rate of chemical reaction (-rA) vs. time at different temperatures to study the
dynamics of the chemical reaction on the same plot.
4. Also plot concentration vs. time graph for different temperatures on the same plot.
5. Finally plot the relationship between (-rA) vs. temperature to see the effect of
temperature on rate of reaction i.e. -rA= f(T).
Observations And Calculations:
Considering reaction 2nd order i.e. n=2
Sr. no Temperature Conc. k -r1 = kCA2
(°C) (M)
NFC IET MULTAN
Chemical Reaction Engineering
Graphs:
Rate of
reaction
Temperature
Theory:
The following topics should be covered by students.
Concept of Arrhenius law.
Knowhow of activation energy, frequency factor and rate constant.
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 10
Fluidized bed reactor
Object:
To study the effect of superficial air velocity on overall heat transfer co-efficient for the
air-steam system in a fluidized bed reactor.
Observations and Calculations:
Packing size =
Thermal conductivity of Aluminum (tube material) = K = 250 KW/mK
Thickness of reactor tube =s= 00016m
Diameter of reactor tube =d= 0.0762
Cross-sectional area of reactor tube = A= (π/4) d2 = 0.785 x (0.0762)2 = 0.00456 m2
Density of air = PM/RT
Superficial air velocity = volumetric flow rate/ cross-sectional area of reactor tube
Mass flow rate= volumetric flow rate x density
Conductive heat transfer coefficient = hcond = K/s =0.25/0.0016 = 16.25W/m2.K
Specific heat of air = Cp=1.006 Kj/kg.K
Heat = Q= mCP∆T
Surface area = As = πdl = 3.14 x 0.0762 x 0.3048 =0.073 m2
Convective heat transfer coefficient = hconv = Q/As∆T
Overall heat transfer coefficient= U= hcond + hconv
NFC IET MULTAN
Chemical Reaction Engineering
Sr. Volumetric Superficial Mass Q=mCP∆T ∆T hconv U
No Flow rate Air flow rate (kW) Tsteam-Tair =Q/As.∆T (W.m2.K)
(L/min) velocity of air (K) (W/m2.K)
(m/sec) (kg/sec)
Theory:
The following topics should be covered by students
Concept of fluidization.
Concept of fluidized bed reactor
Knowhow of superficial air velocity, heat transfer coefficient and Fourier Law.
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 11
Object:
Determine rate of conversion of hydrogen to ethane as a function of time in a tubular
flow reactor.
Equipment:
Tubular flow reactor, stop watch.
Procedure:
1. Prepare the experimental set-up for the proposed experiment.
2. Pre-set the temperature of hot water bath so that it gives a constant reactor wall
temperature throughout the experiment.
3. Fix the feed flow rate and allow it to pass through the reactor before allowing the hot
water to pass through the jacket.
4. Note down the flow rate of feed (gm-moles/sec) and hot water flow rate (cm3/sec)
5. Keep noting the product composition, reactor wall temperature and fixed bed
temperature and hot water inlet and outlet temperatures after every five minutes until
steady state reaches. At this moment, finally record all the parameters.
6. Increase the flow rate of the feed and repeat the procedure.
7. Tabulate the data, as given in table (1).
8. Plot the conversion of hydrogen (yai – yao) as a function of time.
Table (1)
Consumption of hydrogen in reaction as a function of time
Volume of reactor = cm3
0
Reactor wall temperature = C
Mole fraction hydrogen in feed, yai =
NFC IET MULTAN
Chemical Reaction Engineering
Feed rate, F Time, t Mole Mole Catalyst bed
Run (gm- fraction fraction of temperature
No. moles/sec) hydrogen in hydrogen T
product consumed
min sec stream, in reaction, 0C K
yao (yai-yao)
01 (Fixed 0
throughout 5
the run) 10
15
20
02 (Fixed 0
throughout 5
the run) 10
15
20
Theory:
The following topics should be covered by students
Concept of catalytic tubular reactor.
Concept of hydrogenation reaction.
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 12
Fluidized bed reactor
Object:
To study the effect of superficial air velocity on pressure drop in fixed and fluidized
bed conditions and compare their results.
Observations and Calculations:
Packing size=
Thermal conductivity of Aluminum (tube material) = K = 250 kW/m.K
Thickness of reactor tube= 0.0016m
Diameter of reactor tube= d= 0.0762m
Cross-sectional area of reactor tube =A= (π/4)d2 = 0.785 x (0.0762)2 = 0.00456 m2
Density of air= 1.006 KJ/kg.K
Superficial air velocity = volumetric flow rate/cross-sectional area of reactor tube
Pressure drop= Pg/gch
For fixed bed
Sr.no Volumetric Superficial air Difference in Pressure drop
flow rate velocity height (kgF/m2)
(L/min) (m/sec) (m)
NFC IET MULTAN
Chemical Reaction Engineering
For fluidized bed
Sr.no Volumetric Superficial air Difference in Pressure drop
flow rate velocity height (kgF/m2)
(L/min) (m/sec) (m)
Theory:
The following topics should be covered by students
Concept of fluidization.
Concept of fluidized bed reactor
Concept of fixed bed reactor
Understanding of pressure drop
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO.13
Hybrid Reactor
Object:
To study the effect of superficial air velocity on overall heat transfer co-efficient for the
air-steam system in a hybrid reactor.
Observations And Calculations:
Packing size =
Thermal conductivity of Aluminum (tube material) = K = 250 Kw/mK
Thickness of reactor tube =s= 00016m
Diameter of reactor tube =d= 0.0762
Cross-sectional area of reactor tube = A= (π/4) d2 = 0.785 x (0.0762)2 = 0.00456 m2
Density of air = PM/RT
Superficial air velocity = volumetric flow rate/ cross-sectional area of reactor tube
Mass flow rate= volumetric flow rate x density
Conductive heat transfer coefficient = hcond = K/s =0.25/0.0016 = 16.25W/m2.K
Specific heat of air = Cp=1.006 Kj/kg.K
Heat = Q= mCP∆T
Surface area = As = πdl = 3.14 x 0.0762 x 0.3048 =0.073 m2
Convective heat transfer coefficient = hconv = Q/As∆T
Overall heat transfer coefficient= U= hcond + hconv
NFC IET MULTAN
Chemical Reaction Engineering
Sr. Volumetric Superficial Mass Q=mCP∆T ∆T hconv U
No Flow rate Air flow (kW) Tsteam-Tair =Q/As.∆T (W.m2.K)
(L/min) velocity rate of (K) (W/m2.K)
(m/sec) air
(kg/sec)
Theory:
The following topics should be covered by students
Concept of hybridization and hybrid reactor.
Understanding of heat transfer coefficient
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 14
Object:
To determine the rate of reaction in the sulfonation of benzene on a pilot plant.
Procedure:
1. Operate the plant at a temperature of 90 0C until steady state reaches. At this point,
analyze the sample of reaction mixture for Xa, XW and XS.
2. Allow the plant to cool to atmospheric temperature and make it ready for the next run.
3. Operate the plant at temperature of 95 0C, 100 0C and 105 0C and collect sample at
completion of reaction.
4. Analyze the samples in the laboratory and find out the values of Xa, XW and XS.
5. Tabulate the data.
6. Use the formula to calculate the rate of reaction.
Observations And Calculations:
𝑅𝐿 1 1
= 118 (𝑋𝑎 − 2 𝑋𝑤 + 4 𝑋𝑠 )−9.239+5349/𝑇
𝐶𝑎
Concentration of H2SO4 = Ca = (moles/liter)
Run no. Temperature (0C) Absolute Mole fractions
temperature Xa Xw Xs
(K)
Theory:
The following topics should be covered by students
Concept of sulfonation reaction.
Understanding of plate columns, heat exchangers, decanter, drier, centrifugal pump
NFC IET MULTAN
Chemical Reaction Engineering
Learning Outcomes:
NFC IET MULTAN
Chemical Reaction Engineering
EXPERIMENT NO. 15
Object:
To plot conversion of formalin as a function of time.
Procedure:
1. Take 50 gm sample of formalin solution before adding it to the reactor.
2. Calculate formalin in the sample (w1).
3. Operate the plant as discussed in section (3.6).
4. Take sample of reaction mixture after 30 minutes of addition of ammonia to the reactor.
5. Analyze the sample for unconverted formalin (w2).
6. After this, take samples of the reaction mixture (50 gm) after every 10 minutes till the
reaction is complete and analyze the samples for unconvted formalin.
Observations and Calculations:
1. From the analysis of each run, calculate the amount of formalin converted to HMT as
w3 (w3= w1 – w2).
2. Complete table (1) and plot the data as percent formalin converted to HMT as a function
of time.
Table (1)
Conversion of formalin as a function of time
Time Weight of Weight of formalin Weight of Percent
(min) sample (gm) unconverted (gm) formalin formalin
w1 w2 converted (gm) converted
w3= w1 – w2 (w3/w1) x 100
NFC IET MULTAN
Chemical Reaction Engineering
Theory:
The following topics should be covered by students
Properties and uses of hexamethylene tetramine.
Concept of heating and cooling methods in a chemical reactor.
Learning Outcomes:
NFC IET MULTAN
Вам также может понравиться
- Assignment 3Документ5 страницAssignment 3Nabeel BhuttaОценок пока нет
- Brain Stuff PDFДокумент18 страницBrain Stuff PDFKrish Veni100% (1)
- Centrifugal Graph PDFДокумент1 страницаCentrifugal Graph PDFNabeel BhuttaОценок пока нет
- Budget CDLSДокумент2 страницыBudget CDLSNabeel BhuttaОценок пока нет
- Budget CDLSДокумент2 страницыBudget CDLSNabeel BhuttaОценок пока нет
- Registration Form EATДокумент6 страницRegistration Form EATNabeel BhuttaОценок пока нет
- Lecture Notes On Computational Fluid Dynamics: Dan S. Henningson Martin Berggren January 13, 2005Документ115 страницLecture Notes On Computational Fluid Dynamics: Dan S. Henningson Martin Berggren January 13, 2005myrazainalОценок пока нет
- Experiment # 7: Objective of The StudyДокумент1 страницаExperiment # 7: Objective of The StudyNabeel BhuttaОценок пока нет
- Lab02: Arc Command.: Lab Points Scored Lab PerformanceДокумент4 страницыLab02: Arc Command.: Lab Points Scored Lab PerformanceNabeel BhuttaОценок пока нет
- Line Command AutocadДокумент20 страницLine Command AutocadNabeel BhuttaОценок пока нет
- Design and Fabriction of Complex Reactor Combinationof (CSTR, PFR, Tubular & Isothermal Batch Reactor)Документ29 страницDesign and Fabriction of Complex Reactor Combinationof (CSTR, PFR, Tubular & Isothermal Batch Reactor)Julio Antonio Merino CorcioОценок пока нет
- HEC Scholarship Aptitude Test Sample Paper GuideДокумент340 страницHEC Scholarship Aptitude Test Sample Paper GuideMuhammad Faiz Ur Rehman0% (2)
- ASTM D92-05 Standard Test Method For Flash and Fire Points by Cleveland Open Cup Tester PDFДокумент10 страницASTM D92-05 Standard Test Method For Flash and Fire Points by Cleveland Open Cup Tester PDFEduarddo Ravelo NietoОценок пока нет
- ASTM D2500 Cloud Point of Petroleum Products PDFДокумент4 страницыASTM D2500 Cloud Point of Petroleum Products PDFPedro AluaОценок пока нет
- Coal Combustion TechniquesДокумент14 страницCoal Combustion TechniquesNabeel BhuttaОценок пока нет
- Textile EngineeringДокумент14 страницTextile EngineeringNabeel BhuttaОценок пока нет
- Groundtruthtrekking Org PDFДокумент1 страницаGroundtruthtrekking Org PDFNabeel BhuttaОценок пока нет
- Ijshr0004 PDFДокумент9 страницIjshr0004 PDFNabeel BhuttaОценок пока нет
- M.SC - Material Science PDFДокумент48 страницM.SC - Material Science PDFVarun Hm ReddyОценок пока нет
- CGE Greenhouse Gas Inventory Hands-On Training Workshop: Energy SectorДокумент44 страницыCGE Greenhouse Gas Inventory Hands-On Training Workshop: Energy SectoratulsemiloОценок пока нет
- RefractoriesДокумент23 страницыRefractoriesNabeel BhuttaОценок пока нет
- BSc 4th Semester Course Outline for Chemical EngineeringДокумент6 страницBSc 4th Semester Course Outline for Chemical EngineeringNabeel BhuttaОценок пока нет
- Textile EngineeringДокумент14 страницTextile EngineeringNabeel BhuttaОценок пока нет
- Metallurgical & Material EngineeringДокумент6 страницMetallurgical & Material EngineeringNabeel BhuttaОценок пока нет
- Insecticides and Their ApplicationsДокумент133 страницыInsecticides and Their ApplicationsNabeel BhuttaОценок пока нет
- Valve Uses ApplicationsДокумент4 страницыValve Uses ApplicationsMD Shadikul Huq ShezanОценок пока нет
- Fee Expenses For PH.D StudentsДокумент1 страницаFee Expenses For PH.D StudentsNabeel BhuttaОценок пока нет
- BSc 4th Semester Course Outline for Chemical EngineeringДокумент6 страницBSc 4th Semester Course Outline for Chemical EngineeringNabeel BhuttaОценок пока нет
- Term Report CFDДокумент16 страницTerm Report CFDNabeel BhuttaОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Understanding Community Dynamics Through Different PerspectivesДокумент14 страницUnderstanding Community Dynamics Through Different PerspectivesJhener NonesaОценок пока нет
- History of Environmental Protection in IndiaДокумент10 страницHistory of Environmental Protection in IndiaPriyankaJainОценок пока нет
- Demon Magic - Love MagicДокумент44 страницыDemon Magic - Love MagicKama78100% (1)
- Full Download Ethical Obligations and Decision Making in Accounting Text and Cases 4th Edition Mintz Test BankДокумент36 страницFull Download Ethical Obligations and Decision Making in Accounting Text and Cases 4th Edition Mintz Test Banktrichitegraverye1bzv8100% (41)
- Orthogonal Deformation in Ecuador's Eastern AndesДокумент4 страницыOrthogonal Deformation in Ecuador's Eastern AndesAna Chiluisa GuamanОценок пока нет
- The Traveler: Classification and Types of Group TravelersДокумент20 страницThe Traveler: Classification and Types of Group TravelersChin DhoОценок пока нет
- REVIEW INNOVATIONS CE BOARD EXAM MAY 2022 ALVAREZ 1Документ3 страницыREVIEW INNOVATIONS CE BOARD EXAM MAY 2022 ALVAREZ 1Mayya BonaОценок пока нет
- Price List 2021 for CPVC & UPVC Pipes & FittingsДокумент12 страницPrice List 2021 for CPVC & UPVC Pipes & FittingsyashОценок пока нет
- Sistem Slip Gaji Malaysia V1.00Документ2 страницыSistem Slip Gaji Malaysia V1.00suhaimi28Оценок пока нет
- Doodle Business & Consulting Toolkit by SlidesgoДокумент75 страницDoodle Business & Consulting Toolkit by SlidesgoKiki Kiki100% (1)
- High Performance Materials For Passive HouseДокумент102 страницыHigh Performance Materials For Passive HouseLuminita OprutaОценок пока нет
- Mt. Hamiguitan Range Wildlife Sanctuary: Executive SummaryДокумент529 страницMt. Hamiguitan Range Wildlife Sanctuary: Executive SummaryMARSHELLA PONTILLOОценок пока нет
- PDFДокумент470 страницPDFkuzakutuza100% (1)
- Project Management For Managers: Lec - 10 Methods of Project Selection (MCDM - I)Документ18 страницProject Management For Managers: Lec - 10 Methods of Project Selection (MCDM - I)BiswanathMudiОценок пока нет
- BIO 1510 ELISA Lab WorksheetДокумент3 страницыBIO 1510 ELISA Lab WorksheetPaige DarbonneОценок пока нет
- Studi Kasus Pada Perusahaan Manufaktur Di Kawasan Industri Pelabuhan SemarangДокумент12 страницStudi Kasus Pada Perusahaan Manufaktur Di Kawasan Industri Pelabuhan SemarangsherinОценок пока нет
- Discrete Optimization Lecture Notes 5Документ3 страницыDiscrete Optimization Lecture Notes 5BertvdvenОценок пока нет
- 16 Personality Type WorksheetДокумент3 страницы16 Personality Type Worksheetapi-650759265Оценок пока нет
- Journal of Dental Research: Resin-Composite Blocks For Dental CAD/CAM ApplicationsДокумент4 страницыJournal of Dental Research: Resin-Composite Blocks For Dental CAD/CAM Applicationsgerson fabian arangoОценок пока нет
- An Investigation Into The Relationship Between Teaching Methods and Academic Performance of Secondary School Students in NigeriaДокумент61 страницаAn Investigation Into The Relationship Between Teaching Methods and Academic Performance of Secondary School Students in NigeriaUsman Ahmad TijjaniОценок пока нет
- CFD Lecture on Flow Visualization Lines and Governing EquationsДокумент19 страницCFD Lecture on Flow Visualization Lines and Governing EquationsShahzaib Anwar OffОценок пока нет
- DLL Reading StrategiesДокумент3 страницыDLL Reading Strategiesariane galenoОценок пока нет
- 1996 - Tessellation of Trimmed NURB Surfaces PDFДокумент15 страниц1996 - Tessellation of Trimmed NURB Surfaces PDFzxwx1111Оценок пока нет
- Manual Oxygen SensorsДокумент39 страницManual Oxygen SensorsIoanОценок пока нет
- In South-Western Nigeria. African Journal of Plant Science, 3 (9), 210-216Документ2 страницыIn South-Western Nigeria. African Journal of Plant Science, 3 (9), 210-216Ayu CaryutiОценок пока нет
- Non-Linear PDEsДокумент9 страницNon-Linear PDEsPrakritish GhoshОценок пока нет
- Placement Willingness Form For Batch 2020-2022 (Sanjivani MBA)Документ1 страницаPlacement Willingness Form For Batch 2020-2022 (Sanjivani MBA)pramod kakadОценок пока нет
- Anatomy of Flowering PlantsДокумент40 страницAnatomy of Flowering PlantsChaitali DeshmukhОценок пока нет
- A7793a A7797aДокумент1 страницаA7793a A7797aCarlos Rafael Rondon AbreuОценок пока нет
- Plant Trees To Avoid FloodingДокумент5 страницPlant Trees To Avoid FloodingMunting TahananОценок пока нет