Академический Документы
Профессиональный Документы
Культура Документы
Nasal Cell: Septal Squamous Carcinoma: Chart Review and Meta-Analysis
Загружено:
rioОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Nasal Cell: Septal Squamous Carcinoma: Chart Review and Meta-Analysis
Загружено:
rioАвторское право:
Доступные форматы
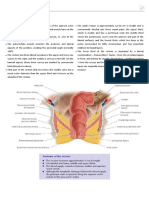
Nasal Septal Squamous Cell Carcinoma:
A Chart Review and Meta-analysis
Michael D. DiLeo, MD; Robert H. Miller, MD; Janet C. Rice, PhD; R. Brent Butcher, MD
Squamous cell carcinoma of the nasal septum oc- reported series with 11 or more patients, for an addi-
curs infrequently and is often misdiagnosed because tional 143 cases.1.2.4-7
its symptoms are similar to everyday rhinologic com-
plaints. The published series have been too small to Although no unique staging system exists, prima-
determine the best form of treatment for this cancer. ry nasal septal squamous cell carcinomas can be sepa-
The authors of this study retrospectively reviewed rated from other lesions of the nasal cavity and
nasal septal squamous cell carcinomas treated at paranasal sinuses by their improved prognosis. Com-
three university-affiliated hospitals over a 30-year pared with lesions of adjacent nasal sites, septal le-
period. Sixteen primary tumors were identified and sions have a better outcome because they present ear-
reviewed for presentation, staging, treatment, recur-
rence patterns, and risk factors. The authors then lier and at a smaller size and because they are more
combined their findings with those of suitable litera- radiosensitive.6 This variable behavior of nasal cavity
ture series and performeda meta-analysisto evaluate lesions is less than adequately accounted for by the
predictors of survival. numerous proposed staging systems that only make
distinctions according to tumor size and extension.7-11
There were too few patients in each stage and Lesions in an advanced state can create even more
treatment group to significantly determine the opti-
mal treatment for nasal septal squamous cell carcino- problems in staging, in that it is difficult to differenti-
ma. However, based on the present study and the lit- ate between the epicenter of the tumor and an area of
erature series, the authors suggest that small lesions extension.
may be confidently treated with either radiation or
surgery and that combined therapy may be reserved Classically, certain occupations (e.g., woodwork-
for more advanced tumors. ing, leathenvorking, nickel refining) and exposures
(e.g., solvents, petroleum products, bakery products,
LARYNGOSCOPE,
106:1218-1222,1996 tobacco) have been identified in relationship to malig-
nant lesions of the nasal cavity and paranasal sinuses.
INTRODUCTION Their contribution to the development of nasal septal
The precise treatment for nasal septal squamous squamous cell carcinomas is unknown, and several se-
cell carcinoma has yet to be determined. This uncer- ries have found little association with these risk fac-
tainty concerning therapy stems from two sources: the t o r ~ .The
~ . ~rarity of these tumors may never allow iso-
rarity of this cancer and the lack of proper therapeutic lation of the inciting events.
trials. Although squamous cell carcinoma accounts for The symptoms of squamous cell carcinoma of the
two-thirds of the primary carcinomas of the nasal sep- nasal septum often are those of everyday rhinologic
tum, these lesions comprise only 9% of malignant tu- complaints. Consequently, patients may delay seeking
mors of the nasal cavity, which, in turn, represent only medical care for up to 6 years."7 Patients most fre-
1%of all human rnalignancies.*a Squamous cell car- quently present with complaints of nasal obstruction,
cinoma of the nasal septum has been reported infre- epistaxis, nasal mass, ulceration, pain, and rhinor-
quently in the literature, with only 97 cases listed rhea.' More advanced disease may present as nasal
through 1978.5 Since 1978, there have been only six swelling, epiphora, diplopia, or palatal ulceration. Un-
fortunately, the diagnosis may be delayed even more if
the nasal cavity is not adequately examined.
Presented a t the Meeting of the Southern Section of the American Common physical findings include mucosal ul-
Laryngological, Rhinological and Otological Society, Inc., Naples, Fla., Janu-
ary 6,1996. ceration, mass lesion, polyp, nasal swelling or deformi-
From the Ikpartment of Otolaryngology-Head and Neck Surgery ty, and septal perforation. 1 Septal carcinoma should
lxi).i)..H . H . M . ) . ' M a n e University School of Medicine, the Department of be strongly suspected in a patient with persistent
Biostatisticb: and EpidemioloLy l J . c . K . ) , Tulane School of Public Health and
Tropical Medicine, and the Department of Otolaryngology-Head and Neck nasal crusting and intermittent bleeding.5 Rarely is a
Surgery ( R . R . H . ) , Ochsner Foundation Hospital, New Orleans. biopsy performed on the first physical contact with a
Reprints not available. patient. 12
Laryngoscope106: October 1996 DiLeo et al.: Nasal Septal Cancer
1218
TABLE I.
Characteristicsof 16 Patients With Primary Nasal Septal Squamous Cell Carcinoma,
Patient No. Sex Age Stage Treatment Recurrence FOIIOW-UP Status
1 F 52 Y T1 Surgery 0 63 mo DNED
2 F 72 Y T1 Radiation Progression 5 rno DWD
3 M 49 Y T1 Radiation Two regional 91 mo ANED
4 M 52 Y T1 N1 Combined 0 6 mo ANED
5 M 68 Y T2 Surgery 0 115rno DNED
6 M 59 Y T2 Surgery 0 26 mo ANED
7 M 64 Y T2 Surgery 0 124mo ANED
8 M 67 Y T2 Surgery 0 70 mo DNED
9 M 50 Y T2 Surgery 0 180 mo DNED
10 F 73 Y T2 Surgery 0 26 rno ANED
11 M 65 Y T2 Radiation 0 240 mo DNED
12 M 70 Y T2 Combined One regional 8 mo DNED
13 M 88 Y T3 Surgery 0 6 mo DNED
14 M 59 Y T3 Surgery Two localhegional 67 mo DWD
15 M 56 Y T3 Combined 0 12 rno ANED
16 F 45 Y T3 Combined 0 54 mo DNED
Combined = both surgery and irradiation: ANED =alive with no evidence of disease:DNED = died with no evidence of disease: DWD = dead with disease.
The literature supports treatment of nasal septal Literature Review and Meta-analysis
squamous cell carcinoma with surgery, radiation, or A search of the English language literature since 1966
combined therapy, and the reported survival rates was performed to identify all series of nasal septal squamous
range from 66% to lOO%.l.",P7 Due to the scant num- cell carcinomas. Only primary tumor series giving individ-
bers of cases, studies have not provided adequate com- ual patient stage, treatment, survival, and disease status
parisons between treatment groups. To extend this were included in the analysis. Tumor stage, treatment type,
limited experience, we performed a retrospective re- and study group were evaluated as predictors of survival us-
view of the charts of patients with squamous cell carci- ing the Cox proportional hazards model.
noma of the nasal septum who were treated at three
university-affiliated hospitals. Suitable literature se- RESULTS
ries were also reviewed and were combined with our
findings in a meta-analysis to determine the optimal Chart Review
treatment for this cancer. Sixteen patients were found to have histologically
confirmed squamous cell carcinoma originating from
MATERIALS AND METHODS the nasal septum (Table I). The 12 male and 4 female
patients had a mean age of 62 years (range: 45 to 88
Retrospective Chart Review years). The time from first symptom to presentation
averaged 12 months (range: 0 to 48 months), and the
The medical records and tumor registries of three uni- most common initial symptom was a nasal mass
versity-affiliated hospitals were searched for the diagnosis (Table 11). The time from the initial physician visit to
of nasal cavity carcinoma in order to identify patients with the diagnosis of squamous cell carcinoma of the nasal
primary nasal septal squamous cell carcinoma. The institu- septum averaged 6 months (range: 0 to 48 months). On
tions were tertiary referral centers, and the cases dated back physical examination, the most common findings were
14,20,and 30 years, depending on the availability of medical nasal ulcerations, masses, septal perforations, and
records and tumor registry data at each location. skin changes.
Only those patients for whom chart notes, surgical re- Tumors were staged as T1 in four patients, T2 in
ports, or pathologic descriptions clearly identified the tumor eight patients, and T3 in four patients. The tumor in
as a primary squamous cell carcinoma of the nasal septum one patient with neck disease was initially thought to
were entered into the study. The charts were reviewed for be an N1 unknown primary, but a T1 septal lesion was
presenting signs and symptoms, delay in presentation and
diagnosis, smoking history, occupation, tumor stage, treat-
subsequently identified. Treatment consisted of surgi-
ment, margins, second primary tumors, recurrences, and cal excision in nine patients, radiation therapy in
survival. Tumors were staged according to the staging sys- three patients, and combined therapy in four patients
tem for carcinoma of the nasal cavity used at the Indiana (Table 111). The total radiation dose ranged from 50 to
University School of Medicine.8 Survival was calculated us- 70 Gy. No neck dissections were performed a t the ini-
ing the Kaplan-Meier method. tial treatment.
Laryngoscope 106: October 1996 DiLeo et at.: Nasal Septal Cancer
1219
TABLE 111.
Patient Series bv Tumor Staae and Treatment.
Treatment
Serieslumor Stage Surgery Radiation Combined
Present series
I 1 2 0
II 6 1 1
111 2 0 3
Young (1979)'
I 1 8 2
"1 ,I
II 0 7 1
111 0 3 0
Fradis, et al. (1993)4
, , , , , , , , , I 0 0 11
0% Combined = both surgery and irradiation.
0 12 24 36 40 0 72 84 08 108 120
Time (Months)
TABLE IV.
Fig. 1. Kaplan-Meier survival curves for a series of 16 patients with
Cox ProDortionalHazards Model for the Combined Series.
nasal septal squamous cell carcinoma.
Variable Chi-SauaredValue PValue
Tumor stage 0.37 0.55
The mean duration of follow-up was 68 months Treatment group 0.76 0.68
(range: 3 to 240 months). The overall 5-year survival Study Group 0.26 0.61
by the Kaplan-Meier method was 66%, and the re-
lapse-free survival was 51% (Fig. 1).
Three known recurrences and one progression of for a planned definitive surgical excision and died with
disease occurred in the group. One patient in the sur- no evidence of disease at 63 months.
gical group, initially with a T3 tumor, had local recur-
rence 19 months after surgery and had localhegional Substantial smoking histories were reported in
disease a t 67 months. This patient died of disease. One 15of the 16 patients, with 11of these histories quanti-
patient in the radiation group, initially with a T1 tu- tated in pack-years for a mean of 56 pack-years. The
mor, had tumor recurrence in the neck at 12 and 53 few occupations that were mentioned included chemi-
months, was surgically salvaged, and was alive with- cal plant worker, street sweeper, house cleaner, and a
out disease a t 91 months. One patient in the com- firefighter who also refinished furniture. Nine second
bined-therapy group, initially with a T2 tumor, had a primary tumors were found in 7 patients, including
recurrence in the neck a t 1month and died of unrelat- leukemia in 2 patients and oral cancer in 2 patients,
ed causes a t 8 months. There was one patient who had with lung, renal, colon, and skin squamous cell and
progression of disease after radiation therapy for a T1 basal cell carcinoma each occurring once.
lesion. Another patient who had a biopsy of a T1 lesion
and topical 5-fluorouracil therapy was lost to follow-up
Meta-analysis
TABLE II. Since 1966,12 articles in the literature have pre-
Symptoms and Physical Findin s in 16 Patients With sented series of patients with primary nasal septal
Nasal Seotal Sauamous 8ell Carcinoma.
Symptoms and Findings Percentage of Patients
squamous cell ~arcinorna.1-7~~.12-1~ Of these, only 2
~ t u d i e s lprovided
.~ adequate information concerning
InitialSymptom
Mass 40%
stage, treatment, survival, and disease status for each
Ulcer 27% individual patient (Table 111). Both series were retro-
Pain 13% spective studies, with one including 22 patients treat-
Nasal obstruction 13% ed primarily with radiation therapy and the other in-
Nasal drainage 7% cluding 11 patients treated with both surgery and
Physical Findings irradiation. When these patients were combined with
Ulcer 56% those in the present series, a total of 49 cases of squa-
Mass 50% mous cell carcinoma of the nasal septum were re-
Perforation 19% viewed.
Skin Changes 19%
Cellulitis 13% Tumor stage, type of treatment, and study group
Nasal deformity 13% were evaluated using a Cox proportional hazards
Edema 13% model. None of the variables was found to be a signifi-
Septal deviation 7% cant predictor of survival (Table IV).
Laryngoscope 106:October 1996 DiLeo et al.: Nasal Septal Cancer
1220
DISCUSSION septal squamous cell carcinoma collected over an 18-
Treatment options for nasal septal squamous cell year period.7 Seven patients underwent surgery, 2 pa-
carcinoma include surgery, radiation, and combined tients received radiation therapy, and 9 patients were
therapy. Localized anterior septal lesions are removed treated with both surgery and irradiation. The ab-
through a lateral rhinotomy incision to obtain ade- solute 5-year survival rate was 66%. Fourteen of these
quate exposure. Wide excision with l-cm margins and patients were smokers. Synchronous primary tumors
the removal of full-thickness mucosa, cartilage, and/or occurred in 17% of patients. On follow-up, the 44% in-
bone is recommended. Early submucosal spread is cidence of neck disease was significantly associated
thought to be enhanced by bony and cartilaginous bar- with primary tumors greater than 2 cm in size. Half of
riers.3 With lesions that advance into the posterior the patients with neck recurrences died of their dis-
septum or the nasal floor, a more extended approach, ease. Due to the high incidence of regional recurrences
such as the Weber-Furguson incision, must be em- and the poor prognosis for these tumors, prophylactic
ployed, along with removal of the involved palate, col- neck irradiation was recommended, along with com-
umella, and medial maxillary wall. For large tumors, bined therapy a t the primary site.
palatal prostheses play an important role in immedi- A 1982 report covered a large series of 58 patients
ate rehabilitation. with septal squamous cell carcinoma who were treat-
In nasal septal squamous cell carcinomas requir- ed over a 61-year period.2 Surgery was used in 69% of
ing excision of all or part of the external nose, immedi- patients, radiation therapy in 5% of patients, and com-
ate reconstruction has been tempered by the known bined therapy in 22% of patients. Of the 58 patients,
indolence of these lesion, and close inspection of the 80% were smokers. The overall 5-year survival was
cavity is required.3 Even in the worst situations, ac- 67%, but no attempt was made to relate survival to
ceptable cosmetic results have been achieved with ad- treatment or stage.
hesive or bone-anchored prosthetics. 1.14 In the present study, the 66% overall 5-year sur-
Radiation therapy for these tumors typically em- vival rate is consistent with the survival rates in other
ploys 60 to 70 Gy to the nasal cavity.eJoJ1 Nasolabial, series with comparable proportions of early- and late-
buccal, and submandibular lymphatics are frequently stage lesions.2.7 However, the small number of pa-
included in the treatment portals. Radiation therapy tients in each tumor staging and treatment group do
is not without risk, and it may result in abnormalities not allow for a n accurate comparison of survival. The
in ciliary activity and the mucous blanket, fibrosis, tis- surgically treated group was the largest, with eight of
sue thickening, and, possibly, chondritis.3 nine patients (one T3 patient recurred locally and re-
gionally) subsequently free of disease. This outcome
The literature review revealed series that support suggests a role for surgery alone in early lesions. The
both radiation therapy and combined therapy as pri- inordinate delay from initial symptoms to presenta-
mary treatment modalities for nasal septal squamous tion and diagnosis emphasizes the need for a thorough
cell carcinomas. However, due to the paucity of pa- examination in patients with chronic, persistent rhi-
tients with these lesions, adequately large treatment nologic complaints.
groups have not been assembled to establish optimal
therapy. In a 1993 review of 11patients with septal in- The known 94% incidence of substantial smoking
vasive squamous cell carcinomas,* all lesions were histories in the present series strengthens the implica-
stage I and were treated with combined surgery and tions of several other studies that tobacco is an etiolog-
radiation. There were only two heavy smokers in the ic factor in the disease process.2~7This relationship is
group, and no occupational exposures were identified. h r t h e r intensified by the fact that two of the patients
All patients were free of disease 2.5 to 14.0 years after had oral second primary tumors, suggesting possible
treatment. field cancerization as the basis of disease.
In a 1992 review of 14 patients collected over a 17- In the past, the reconstruction of externally de-
year period,6 the vast majority of lesions were stage I forming excisions was delayed due to the indolent na-
and received external-beam or interstitial radiation ture of septal tumors and the need for cavity inspec-
therapy. Only two tumors were treated with combined t i ~ nIn
. ~the present study, only one surgical case was
surgery and irradiation. The 5-year overall survival known to have a local recurrence. Such a low rate of lo-
rate was 92% after surgical salvage was performed in cal recurrence should not deter primary reconstruc-
4 patients for local or regional recurrences. Two of the tion, and nasal endoscopes make follow-up easier.
8 patients who did not receive prophylactic neck irra-
Meta-analysis is a useful tool for achieving a con-
diation sustained regional recurrences, as compared sensus among a group of patient series when there is
with none of the 6 patients who had neck irradiation.
inadequate individual experience for analysis. Howev-
With these favorable results, the investigators strong-
er, due to the small sample sizes in each treatment and
ly recommended primary radiation therapy for early
stage subgroup and due to the large number of pa-
lesions of the nasal septum.
tients still alive a t the time of final follow-up, the pres-
A 1984 report discussed 18 patients with nasal ent meta-analysis fails to achieve significance. Also,
Laryngoscope 106:October 1996 DiLeo et at.: Nasal Septa1Cancer
1221
because of the rarity of this lesion and the small indi- 3. McGuirt WF, Thompson JN. Surgical approaches to malignant
vidual treatment groups collected over decades, such tumors of the nasal septum. Laryngoscope. 1984;94:1045-
an analysis is plagued by failure of patient randomiza- 1049.
4. Fradis M, Podoshin L, Gertner R, et al. Squamous cell carcino-
tion and inconsistency of treatment methods. A multi- ma ofthe nasal septum mucosa. Ear Nose Throat J . 1993;72:
institutional protocol would more effectively address 217-221.
the best mode of treatment. 5. Weimert TA, Batsakis J G , Rice DH. Carcinomas of the nasal
septum. JLaryngol Otol. 1978;92:209-213.
CONCLUSION 6. Ang KK, Jiang GL, Frankenthaler RA, e t al. Carcinomas of the
nasal cavity. Radiother Oncol. 1992;24:163-168.
The results of the literature series and the pres- 7. LeLiever WC, Bailey BJ, Griffths C. Carcinoma of the nasal
ent study suggest that either radiation therapy or sur- septum. Arch Otolaryngol. 1984;110:748-751.
gery is effective for the treatment of early-stage le- 8. Shidnia H, Hartsough AB, Weisberger E, et 31.Epithelial carci-
sions of primary squamous cell carcinoma of the nasal noma of the nasal fossa. L A R Y N ~ ~ ~ ~~~PE.
1987;97:717-723,
septum. More advanced lesions require the combined 9. Badib AO, Kurohara SS, Webster J H , e t al. Treatment of can-
efforts of surgery and radiation therapy. Because of cer of the nasal cavity. Am J Roentgenol. 1969;106:824-830,
the poor prognosis of a regional recurrence, prophylac- 10. Hawkins RB, Wynstra J H , Pilepich MV, et al. Carcinoma of the
tic radiation therapy for the neck may be beneficial in nasal cavity-results of primary and adjuvant radiotherapy.
more advanced tumors. The usefulness of prophylactic Znt JRadiat Oncol Biol Phys. 1988;15:1129-1133.
11. Parsons JT, Mendenhall WM, Mancuso AA, et al. Malignant
neck dissection has not been examined. The delay in
tumors of the nasal cavity and ethmoid and sphenoid sinus-
diagnosis and the treatable nature of early lesions un- es. Znt JRadiat Oncol BiolPh.ys. 1988;14:11-22.
derscore the importance of a thorough examination in 12. Yarington CT, Jaquiss GW, Sprinkle PM. Carcinoma of the
patients with chronic rhinologic complaints. nose and nasal septum: treatment and reconstruction. Zhans
A m Acad Ophthalmol Otolaryngol. 1969;73:1178-1183.
BIBLIOGRAPHY 13. Lyons GD. Squamous cell carcinoma ofthe nasal septum. Arch
Otolaryrigol. 1969;89:585-587.
1. Young JR. Malignant tumours of the nasal septum. J Laryngol 14. Harrision DFN. Total rhinectomy-a worthwhile operation?
Otol. 1979;93:817-832. J Laryngol Otol. 1982;96: 1113-1123.
2. Beatty CW, Pearson BW, Kern EB. Carcinoma ofthe nasal sep- 15. Whitcomb WP. Radiation therapy of carcinoma of the anterior
tum: experience with 85 cases. Otolaryngol Head Neck Surg. nasal cavity. Am JRoentgenol. 1969;105:550-554.
1982;90:90-94.
Laryngoscope 106:October 1996 DiLeo et al.: Nasal Septa1Cancer
1222
Вам также может понравиться
- Hennessey 2013Документ4 страницыHennessey 2013FarihinternaОценок пока нет
- Synchronous Squamous Cell Carcinoma of The Lip and Nasopharyngeal Carcinoma - A Rare Case Report.Документ4 страницыSynchronous Squamous Cell Carcinoma of The Lip and Nasopharyngeal Carcinoma - A Rare Case Report.International Journal of Innovative Science and Research Technology100% (1)
- Nasopharyngeal Carcinoma: Imaging Features of Unusual Cancer in ChildrenДокумент5 страницNasopharyngeal Carcinoma: Imaging Features of Unusual Cancer in ChildrenDevanti EkaОценок пока нет
- Paranasal Sinuses Malignancies: A 12-Year Review of Clinical CharacteristicsДокумент5 страницParanasal Sinuses Malignancies: A 12-Year Review of Clinical CharacteristicsRidho RifhansyahОценок пока нет
- 184Документ7 страниц184Benedetta GuarinoОценок пока нет
- Paraneoplastic Syndromes Associated With LaryngealДокумент15 страницParaneoplastic Syndromes Associated With Laryngealandre halimОценок пока нет
- Surgical Education: Neck Dissection: Shaheel Chummun, N.R. Mclean, Maniram RagbirДокумент14 страницSurgical Education: Neck Dissection: Shaheel Chummun, N.R. Mclean, Maniram RagbirpaulineОценок пока нет
- Caso Carcinoma Adenoide QuisticoДокумент6 страницCaso Carcinoma Adenoide QuisticoCarballo GiovannyОценок пока нет
- Nasal and Paranasal Sinus PathologiesДокумент7 страницNasal and Paranasal Sinus Pathologiespunct_org3256Оценок пока нет
- Management of Neck Dissection Complications in Head and Neck CancersДокумент5 страницManagement of Neck Dissection Complications in Head and Neck CancersDanilo RetanaОценок пока нет
- 5608-Article Text-20048-1-10-20210319Документ4 страницы5608-Article Text-20048-1-10-20210319Muhammad IzehagaОценок пока нет
- PIIS0360301622000049Документ2 страницыPIIS0360301622000049FОценок пока нет
- Tonsillectomy Helps Identify Unknown Primary TumorsДокумент4 страницыTonsillectomy Helps Identify Unknown Primary TumorsMuhammad AbdillahОценок пока нет
- Challenges in The Management of Inverted Papilloma: A Review of 72 Revision CasesДокумент7 страницChallenges in The Management of Inverted Papilloma: A Review of 72 Revision CasesKarina AlvearОценок пока нет
- 11.nasopharyngealcarcinoma NeckДокумент4 страницы11.nasopharyngealcarcinoma Neckwaleed MagidОценок пока нет
- Afp 20021115 P 1882Документ5 страницAfp 20021115 P 1882PPDS THT-KL USKОценок пока нет
- 12 Juvenile NasopharyngealДокумент5 страниц12 Juvenile NasopharyngealkhairanifahmaОценок пока нет
- Nasal Pyramid Skin's Adenosquamous Carcinoma A Rare Case ReportДокумент3 страницыNasal Pyramid Skin's Adenosquamous Carcinoma A Rare Case ReportInternational Journal of Innovative Science and Research TechnologyОценок пока нет
- Barrs 2001Документ22 страницыBarrs 2001AshokОценок пока нет
- Unknown Primary Cancer of The Head and Neck - A MultidisciplinaryДокумент10 страницUnknown Primary Cancer of The Head and Neck - A MultidisciplinaryFreddy Eduardo Navarro BautistaОценок пока нет
- Incidence of Small Lymph Node Metastases in Patients With Nasopharyngeal Carcinoma: Clinical Implications For Prognosis and TreatmentДокумент7 страницIncidence of Small Lymph Node Metastases in Patients With Nasopharyngeal Carcinoma: Clinical Implications For Prognosis and TreatmentAdelin Luthfiana FajrinОценок пока нет
- BIBL 12 Liver MetastasisДокумент3 страницыBIBL 12 Liver Metastasisstoia_sebiОценок пока нет
- Acceptedpaper - A Rare Case Choanal Stenosis Bilateral in Nasopharyngeal Carcinoma Patient With Endoscopic Surgery Management - DR - Yulialdi Bimanto Heryanto Putra - Yulialdi Bimanto Heryanto PutraДокумент7 страницAcceptedpaper - A Rare Case Choanal Stenosis Bilateral in Nasopharyngeal Carcinoma Patient With Endoscopic Surgery Management - DR - Yulialdi Bimanto Heryanto Putra - Yulialdi Bimanto Heryanto PutraRika Sartyca IlhamОценок пока нет
- A Systematic Review of Sinonasal Oncocytomas and Oncocytic Carcinomas: Diagnosis, Management, and Technical ConsiderationsДокумент11 страницA Systematic Review of Sinonasal Oncocytomas and Oncocytic Carcinomas: Diagnosis, Management, and Technical ConsiderationsInes Camilla PutriОценок пока нет
- Mucosal Melanoma of Head & Neck 32-Year StudyДокумент5 страницMucosal Melanoma of Head & Neck 32-Year StudyWahyu JuliandaОценок пока нет
- CA Sinonasal PDFДокумент2 страницыCA Sinonasal PDFGek DewiОценок пока нет
- 17-Potential Medicolegal RisksДокумент1 страница17-Potential Medicolegal RisksSUSANОценок пока нет
- Microsurgery For Eyelid Margin Tumors - OphthaДокумент2 страницыMicrosurgery For Eyelid Margin Tumors - OphthaАнагаахын ОрчуулгаОценок пока нет
- The Management of Sinonasal Inverted Papilloma: Our ExperienceДокумент7 страницThe Management of Sinonasal Inverted Papilloma: Our Experiencesri ayu lestari wulandariОценок пока нет
- An Unusual Presentation of Swelling in The Palate-An Interesting Case ReportДокумент3 страницыAn Unusual Presentation of Swelling in The Palate-An Interesting Case ReportInternational Journal of Innovative Science and Research TechnologyОценок пока нет
- Nasogpangeal AdenoidДокумент6 страницNasogpangeal AdenoidSanggiani Diah AuliaОценок пока нет
- Malignant Melanoma of the External Ear Prognosis Linked to Definitive Wide ExcisionДокумент4 страницыMalignant Melanoma of the External Ear Prognosis Linked to Definitive Wide ExcisionNur Aisyah Soedarmin IieychaОценок пока нет
- Meanma Maligna PDFДокумент4 страницыMeanma Maligna PDFNur Aisyah Soedarmin IieychaОценок пока нет
- 6 Neck DissectionДокумент9 страниц6 Neck DissectionAnne MarieОценок пока нет
- 6324-Article Text-21760-1-10-20210620Документ3 страницы6324-Article Text-21760-1-10-20210620Fritzienico BaskoroОценок пока нет
- Head and Neck Mucosal MelanomaДокумент5 страницHead and Neck Mucosal MelanomaHAMIDОценок пока нет
- Radiological Abnormalities of Paranasal Sinuses in Asthmatics With RhinitisДокумент3 страницыRadiological Abnormalities of Paranasal Sinuses in Asthmatics With RhinitiswisdomatixОценок пока нет
- 960-Article Text-3578-3-10-20190611Документ3 страницы960-Article Text-3578-3-10-20190611Lionel IboyОценок пока нет
- Jomfp 25 58Документ3 страницыJomfp 25 58Gisela LalitaОценок пока нет
- Olfactory Groove MeningiomaДокумент16 страницOlfactory Groove Meningiomaclaudio RivasОценок пока нет
- 0 Clinical Original Contribution: J. FRCR, N. JДокумент4 страницы0 Clinical Original Contribution: J. FRCR, N. JmawarmelatiОценок пока нет
- Nasal Alar Schwannoma An Unusual Case ReportДокумент3 страницыNasal Alar Schwannoma An Unusual Case Reportanitaabreu123Оценок пока нет
- Management of Nasolabial Cysts by Transnasal Endoscopic MarsupializationДокумент8 страницManagement of Nasolabial Cysts by Transnasal Endoscopic Marsupializationfk06Оценок пока нет
- Sinonasal Non-Small Cell Neuroendocrine Carcinoma The Validity of Histological Grading Case Report and A Review of The LiteratureДокумент16 страницSinonasal Non-Small Cell Neuroendocrine Carcinoma The Validity of Histological Grading Case Report and A Review of The Literaturenurul hidayahОценок пока нет
- JNS Role of Electrophysiology in Guiding Near Total Resection For Preservation of Facial Nerve Function in The Surgical Treatment of Large Vestibular SchwannomasДокумент8 страницJNS Role of Electrophysiology in Guiding Near Total Resection For Preservation of Facial Nerve Function in The Surgical Treatment of Large Vestibular SchwannomasAlejandro CheritОценок пока нет
- F0605yДокумент6 страницF0605ySetiaty PandiaОценок пока нет
- Bonnie Vorasubin, MD Arthur Wu, MD Chi Lai, MD Elliot Abemayor, MD, PHDДокумент1 страницаBonnie Vorasubin, MD Arthur Wu, MD Chi Lai, MD Elliot Abemayor, MD, PHDmandible removerОценок пока нет
- Zinc Oxide Nanoparticles Induce Toxicity inДокумент10 страницZinc Oxide Nanoparticles Induce Toxicity inLoredana VoiculescuОценок пока нет
- Comparative Study Between Conventional MicrolaryngealДокумент7 страницComparative Study Between Conventional MicrolaryngealTeuku Ahmad HasanyОценок пока нет
- Imaging of Palatal LumpsДокумент11 страницImaging of Palatal LumpsChavdarОценок пока нет
- Case Report: Matin Ghazizade, Mahboobe Asadi and Pouyan DarbaniДокумент4 страницыCase Report: Matin Ghazizade, Mahboobe Asadi and Pouyan DarbanirizkiaОценок пока нет
- Skullbasesurg00008 0039Документ5 страницSkullbasesurg00008 0039Neetu ChadhaОценок пока нет
- A Case of Nasopalatine Dust Cyst: Presentation, Diagnosis and ManagementДокумент4 страницыA Case of Nasopalatine Dust Cyst: Presentation, Diagnosis and ManagementKertamayaОценок пока нет
- Mendenhall Elective Neck Irradiation, Causes of FailureДокумент6 страницMendenhall Elective Neck Irradiation, Causes of FailureSanjog SinghОценок пока нет
- Oral Cavity and Lip Cancer: United Kingdom National Multidisciplinary GuidelinesДокумент7 страницOral Cavity and Lip Cancer: United Kingdom National Multidisciplinary Guidelineskepipranave-9084Оценок пока нет
- 538-Article Text-7432-1-10-20200805Документ8 страниц538-Article Text-7432-1-10-20200805Ankita GuravОценок пока нет
- Evaluation and Management of Neck MassesДокумент38 страницEvaluation and Management of Neck MassesShaxawan Mahmood AliОценок пока нет
- Facial Nerve Function After Partial Superficial Parotidectomy: An 11-Year Review (1987-1997)Документ4 страницыFacial Nerve Function After Partial Superficial Parotidectomy: An 11-Year Review (1987-1997)KaranPadhaОценок пока нет
- Update On Nasopharyngeal Carcinoma: Ó Humana 2007Документ6 страницUpdate On Nasopharyngeal Carcinoma: Ó Humana 2007Zakia AjaОценок пока нет
- Eustachian Tube PDFДокумент204 страницыEustachian Tube PDFFiona ApriliaОценок пока нет
- 1 RajeshДокумент4 страницы1 RajeshrioОценок пока нет
- Association Between Symptomatic Deviated Nasal Septum and Sinusitis: A Prospective StudyДокумент8 страницAssociation Between Symptomatic Deviated Nasal Septum and Sinusitis: A Prospective StudyYosie Yulanda PutraОценок пока нет
- Association Between Symptomatic Deviated Nasal Septum and Sinusitis: A Prospective StudyДокумент8 страницAssociation Between Symptomatic Deviated Nasal Septum and Sinusitis: A Prospective StudyYosie Yulanda PutraОценок пока нет
- Maranho 2017Документ7 страницMaranho 2017rioОценок пока нет
- Relation Between Deviated Nasal Septum and Paranasal Sinus PathologyДокумент5 страницRelation Between Deviated Nasal Septum and Paranasal Sinus PathologyrioОценок пока нет
- Nasal Septal Deviation Reduces Ethmoid Sinus SizeДокумент5 страницNasal Septal Deviation Reduces Ethmoid Sinus SizerioОценок пока нет
- Lyle 2016Документ7 страницLyle 2016rioОценок пока нет
- Lyle 2016Документ7 страницLyle 2016rioОценок пока нет
- Ajr 12 8494Документ12 страницAjr 12 8494rioОценок пока нет
- Breast Cancer Prevention: Saint Louis University School of Nursing Research JournalДокумент3 страницыBreast Cancer Prevention: Saint Louis University School of Nursing Research JournalDale DeocaresОценок пока нет
- Glimmers of Hope For Targeting Oncogenic KRAS-G12D: Cancer Gene TherapyДокумент3 страницыGlimmers of Hope For Targeting Oncogenic KRAS-G12D: Cancer Gene Therapychato law officeОценок пока нет
- TrafficДокумент9 страницTrafficMelanie LaginanОценок пока нет
- Tumor Pathology AtlasДокумент1 353 страницыTumor Pathology AtlaskaterenchukigorОценок пока нет
- Cancer Epidemiology Assignment Charity NewДокумент8 страницCancer Epidemiology Assignment Charity NewSummer LarinОценок пока нет
- Plasma CutterДокумент22 страницыPlasma CutterDale Beshara100% (2)
- Carcinogen - WikipediaДокумент13 страницCarcinogen - WikipediamrkuroiОценок пока нет
- Rectal CarcinomaДокумент7 страницRectal CarcinomaEbrahim Adel Ali AhmedОценок пока нет
- CA Stomach OverviewДокумент84 страницыCA Stomach OverviewDr Shafiq Ahmad ChughtaiОценок пока нет
- Anal CancerДокумент36 страницAnal CancerBurhan MinervaОценок пока нет
- Worldwide Burden of Colorectal Cancer: A ReviewДокумент5 страницWorldwide Burden of Colorectal Cancer: A ReviewRomário CerqueiraОценок пока нет
- Jurnal Tirosin Kinase 4Документ13 страницJurnal Tirosin Kinase 4Maya SariОценок пока нет
- 2nd Announcement 24 Feb 2020 - 1Документ7 страниц2nd Announcement 24 Feb 2020 - 1reza pahlevi zОценок пока нет
- Resume: Hema Latha KoyeladaДокумент3 страницыResume: Hema Latha KoyeladaSai CharanОценок пока нет
- 2010 (Review) Plant Natural Products in Anticancer Drug Discovery PDFДокумент11 страниц2010 (Review) Plant Natural Products in Anticancer Drug Discovery PDFAmrita PanigrahiОценок пока нет
- Ijramt - 3 3 11Документ4 страницыIjramt - 3 3 11Fayomi JosephОценок пока нет
- ColonosДокумент8 страницColonosMuhammad Bin Abdul AzizОценок пока нет
- Types of CancerДокумент10 страницTypes of CancerStudies 123Оценок пока нет
- Bi Rads 151130171211 Lva1 App6891 PDFДокумент70 страницBi Rads 151130171211 Lva1 App6891 PDFMohammad Ahmad AyasrahОценок пока нет
- RENAL ScoreДокумент5 страницRENAL ScoreMurilo CamposОценок пока нет
- Asian Hps ReportДокумент145 страницAsian Hps ReportUMRATUN HAYATIОценок пока нет
- Prometric Fall 2021Документ15 страницPrometric Fall 2021Abdul Hamid Al FarsiОценок пока нет
- Laporan Penyakit Tidak Menular (PTM) PuskesmasДокумент2 страницыLaporan Penyakit Tidak Menular (PTM) PuskesmasCostafierra AgОценок пока нет
- Rapidplan™ Knowledge-Based Planning: BrainДокумент5 страницRapidplan™ Knowledge-Based Planning: BrainolgaОценок пока нет
- Campbell-Walsh Urology, 12th Edition 1Документ21 страницаCampbell-Walsh Urology, 12th Edition 1NICOLE CARPIO MALAGAОценок пока нет
- Application Form - Essay Prize 2020Документ4 страницыApplication Form - Essay Prize 2020John SmithОценок пока нет
- B-ALL Prediction Using Computer Vision (TensorFlow)Документ18 страницB-ALL Prediction Using Computer Vision (TensorFlow)bipra.patra24Оценок пока нет
- Total Laryngectomy: Technique: Harvey M. Tucker, MDДокумент3 страницыTotal Laryngectomy: Technique: Harvey M. Tucker, MDKumaran Bagavathi RagavanОценок пока нет
- Oral Cancer Grading SystemДокумент42 страницыOral Cancer Grading SystemMadhura ShekatkarОценок пока нет
- Soft Tissue Tumors New Plan 1445Документ32 страницыSoft Tissue Tumors New Plan 1445Asem AlhazmiОценок пока нет