Академический Документы
Профессиональный Документы
Культура Документы
Amer Ebrahim Hamad PDF
Загружено:
Fahad S. EdhamОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Amer Ebrahim Hamad PDF
Загружено:
Fahad S. EdhamАвторское право:
Доступные форматы
وزارة التعليم العالي والبحث العلمي
جامعة تكريت
كلية الهندسة
قسم الهندسة الكيمياوية
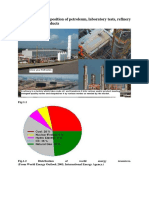
Paraffin and Paraffin Wax
اعداد الطالب :عامر ابراهيم حمد
بأشراف :الدكتور ايسر طالب جارالله
تاريخ تقديم التقرير 0202/6/8 :
introduction:
Paraffins, also known as alkanes, are saturated compounds
that have the general formula CnH2n+2, where n is the
number of carbon atoms. The simplest alkane is methane
(CH4), which is also represented as C1. Normal paraffins
(n-paraffins or n-alkanes) are unbranched straightchain
molecules. Each member of these paraffins differs from
the next higher and the next lower member by a –CH2–
group called a methylene group (Table 2.2). They have
similar chemical and physical properties, which change
gradually as carbon atoms are added to the chain.
Isoparaffins (or isoalkanes) are branched-type
hydrocarbons that exhibit structural isomerization.
Structural isomerization occurs when two molecules have
the same atoms but different bonds. In other words, the
moleculeshave the same formulas but different
arrangements of atoms, known as isomers. Butane and all
succeeding alkanes can exist as straight-chain molecules
(n-paraffins) or with a branched-chain structure
(isoparaffins).
Paraffin Wax:
Paraffin wax (or petroleum wax) is a soft colorless solid
derived from petroleum, coal or shale oil that consists of a
mixture of hydrocarbon molecules containing between
twenty and forty carbon atoms. It is solid at room
temperature and begins to melt above approximately 37
°C (99 °F), and its boiling point is above 370 °C (698 °F).
Common applications for paraffin wax include lubrication,
electrical insulation, and candles; dyed paraffin wax can
be made into crayons. It is distinct from kerosene and
other petroleum products that are sometimes called
paraffin. Un-dyed, unscented paraffin candles are
odorless and bluish-white. Paraffin wax was first created
by Carl Reichenbach in Germany in 1830 and marked a
major advancement in candlemaking technology, as it
burned more cleanly and reliably than tallow candles and
was cheaper to produce. In chemistry, paraffin is used
synonymously with alkane, indicating hydrocarbons with
the general formula CnH2n+2. The name is derived from
Latin parum ("barely") + affinis, meaning "lacking affinity"
or "lacking reactivity", referring to paraffin's unreactive
nature
Properties:
Paraffin wax was first created in 1830 by the German
chemist Karl von Reichenbach when he tried to develop
the means to efficiently separate and refine the waxy
substances naturally occurring in petroleum. Paraffin
represented a major advance in the candlemaking
industry because it burned more cleanly and reliably and
was cheaper to manufacture than any other candle fuel.
Paraffin wax initially suffered from a low melting point;
however, this shortcoming was later remedied by the
addition of harder stearic acid. The production of paraffin
wax enjoyed a boom in the early 20th century as a result
of the growth of the meatpacking and oil industries which
created paraffin and stearic acid as byproducts.
Manufacturing:
The feedstock for paraffin is slack wax, which is a mixture
of oil and wax, a byproduct from the refining of
lubricating oil. The first step in making paraffin wax is to
remove the oil (de-oiling or de-waxing) from the slack
wax. The oil is separated by crystallization. Most
commonly, the slack wax is heated, mixed with one or
more solvents such as a ketone and then cooled. As it
cools, wax crystallizes out of the solution, leaving only oil.
This mixture is filtered into two streams: solid (wax plus
some solvent) and liquid (oil and solvent). After the
solvent is recovered by distillation, the resulting products
are called "product wax" (or "press wax") and "foots oil".
The lower the percentage of oil in the wax, the more
refined it is considered (semi-refined versus fully refined).
The product wax may be further processed to remove
colors and odors. The wax may finally be blended
together to give certain desired properties such as melt
point and penetration. Paraffin wax is sold in either liquid
or solid form.
Applications:
In industrial applications, it is often useful to modify the
crystal properties of the paraffin wax, typically by adding
branching to the existing carbon backbone chain. The
modification is usually done with additives, such as EVA
copolymers, microcrystalline wax, or forms of
polyethylene. The branched properties result in a
modified paraffin with a higher viscosity, smaller
crystalline structure, and modified functional properties.
Pure paraffin wax is rarely used for carving original
models for casting metal and other materials in the lost
wax process, as it is relatively brittle at room temperature
and presents the risks of chipping and breakage when
worked. Soft and pliable waxes, like beeswax, may be
preferred for such sculpture, but "investment casting
waxes," often paraffin-based, are expressly formulated for
the purpose. In a histology or pathology laboratory,
paraffin wax is used to impregnate tissue prior to
sectioning thin samples of tissue. Water is removed from
the tissue through ascending strengths of alcohol (75% to
absolute) and the tissue is cleared in an organic solvent
such as xylene. The tissue is then placed in paraffin wax
for a number of hours and then set in a mold with wax to
cool and solidify; sections are then cut on a microtome.
Other uses:
Candle-making
Wax carving
Bicycle chain lubrication
Coatings for waxed paper or cloth
Food-grade paraffin wax:
o Shiny coating used in candy-making; although edible,
it is nondigestible, passing through the body without
being broken down
o Coating for many kinds of hard cheese, like Edam
cheese
o Sealant for jars, cans, and bottles
o Chewing gum additive
Investment casting
Anti-caking agent, moisture repellent, and dustbinding
coatings for fertilizers
Agent for preparation of specimens for histology
Bullet lubricant – with other ingredients, such as olive oil
and beeswax
Phlegmatizing agent, commonly used to
stabilise/desensitize high explosives such as RDX
Crayons
Solid propellant for hybrid rocket motors[22][23]
Component of surfwax, used for grip on surfboards
in surfing
Component of glide wax, used on skis and snowboards
Friction-reducer, for use on handrails and cement
ledges, commonly used in skateboarding
Вам также может понравиться
- Paraffin WaxДокумент5 страницParaffin WaxJoseph0% (1)
- Paraffin - Wikipedia, The Free EncyclopediaДокумент4 страницыParaffin - Wikipedia, The Free Encyclopediaglassyglass0% (1)
- Chapter 3 Wax Processing and PurificationДокумент40 страницChapter 3 Wax Processing and Purificationjiva100% (8)
- Experiment For Base Plate Dental Wax (PTDC) 2014 - LITERATURE STUDY PDFДокумент32 страницыExperiment For Base Plate Dental Wax (PTDC) 2014 - LITERATURE STUDY PDFdentalinosОценок пока нет
- Dental Waxes 1Документ26 страницDental Waxes 1Jyoti Tripathi100% (1)
- Waxes Are A Diverse Class of Organic Compounds That Are Hydrophobic, MalleableДокумент5 страницWaxes Are A Diverse Class of Organic Compounds That Are Hydrophobic, MalleableAmmar SiddiquiОценок пока нет
- WaxДокумент6 страницWaxSuiko22Оценок пока нет
- Shoe PolishДокумент5 страницShoe Polishsuleman20590% (10)
- Petroleum Technology-Part I: Terminology & Classification of PetroleumДокумент1 страницаPetroleum Technology-Part I: Terminology & Classification of PetroleumgamadiyaОценок пока нет
- Petroleum WaxesДокумент18 страницPetroleum WaxesChandra Kant100% (1)
- Petroleum WaxesДокумент18 страницPetroleum WaxesChandra KantОценок пока нет
- Carnauba WaxДокумент5 страницCarnauba WaxsimilcemalcemilОценок пока нет
- Petroleum Origin & ManufacturingДокумент42 страницыPetroleum Origin & ManufacturingEmilien HAINGONIRINAОценок пока нет
- Classification of Crude OilДокумент6 страницClassification of Crude OilSultana AlmansooriОценок пока нет
- Article-106-Spc Waxes Used in The Cosmetics IndustryДокумент3 страницыArticle-106-Spc Waxes Used in The Cosmetics IndustryVictor Lopez100% (1)
- Candles: See Also Waxes, Chap. 4.7Документ2 страницыCandles: See Also Waxes, Chap. 4.7jaimeОценок пока нет
- 1) Characteristics of Petroleum and Its Products.Документ31 страница1) Characteristics of Petroleum and Its Products.SATENDRA TIWARIОценок пока нет
- Petroleum Products: Submitted To: Submitted byДокумент25 страницPetroleum Products: Submitted To: Submitted byMNIT Literary SocietyОценок пока нет
- Introduction To RefineryДокумент6 страницIntroduction To RefinerydyarОценок пока нет
- Lecture 02Документ16 страницLecture 02Touseef IsmailОценок пока нет
- 5.dental Waxes 14Документ14 страниц5.dental Waxes 14Chandra Babu0% (1)
- Dental WaxesДокумент67 страницDental WaxesRicha GujrathiОценок пока нет
- Unit 3 Speciality Products - 2Документ12 страницUnit 3 Speciality Products - 2prathamesh singhОценок пока нет
- Kiến Thức Hóa DầuДокумент364 страницыKiến Thức Hóa DầuTu LaiОценок пока нет
- Unit 2 Chemistry-Crude Oil and AmmoniaДокумент40 страницUnit 2 Chemistry-Crude Oil and AmmoniacrystalОценок пока нет
- Crude Oil The Black GoldДокумент33 страницыCrude Oil The Black GoldOMED gardiОценок пока нет
- KerosenДокумент20 страницKerosenRizwan FaridОценок пока нет
- Chapter No 1Документ18 страницChapter No 1Ali AhsanОценок пока нет
- Chapter-5 FuelДокумент27 страницChapter-5 FueljeetОценок пока нет
- Basics of Refinery1Документ15 страницBasics of Refinery1Sunil PatanwadiyaОценок пока нет
- Dental WaxesДокумент49 страницDental Waxeschoudharyishita163Оценок пока нет
- This Chapter Includes Data About Petroleum and Corrosion in GeneralДокумент25 страницThis Chapter Includes Data About Petroleum and Corrosion in GeneralFadzli KhirОценок пока нет
- Waxy CrudesДокумент1 страницаWaxy CrudesAkash BodekarОценок пока нет
- Dental WaxesДокумент18 страницDental WaxesrajaniОценок пока нет
- BYK B-G1 Wax ENДокумент8 страницBYK B-G1 Wax ENXuân Giang NguyễnОценок пока нет
- CNSLДокумент18 страницCNSLrafeekОценок пока нет
- Varnish: Varnish Is A Clear Transparent Hard Protective Finish or Film. Varnish Has Little or NoДокумент7 страницVarnish: Varnish Is A Clear Transparent Hard Protective Finish or Film. Varnish Has Little or NoRadost Galonja KrtinićОценок пока нет
- Petroleum Naphtha - Wikipedia PDFДокумент4 страницыPetroleum Naphtha - Wikipedia PDFSauptik DattaОценок пока нет
- Petroleum Refinery Powerpoint SlideДокумент22 страницыPetroleum Refinery Powerpoint SlideBharat BajajОценок пока нет
- Crude Oil (Petroleum) : Module 3: Industry and The EnvironmentДокумент8 страницCrude Oil (Petroleum) : Module 3: Industry and The EnvironmentAnonymous Xk1QVIpPJОценок пока нет
- Types: For Other Uses, See Wax (Disambiguation)Документ1 страницаTypes: For Other Uses, See Wax (Disambiguation)glh00Оценок пока нет
- AT6504 AFL Notes PDFДокумент52 страницыAT6504 AFL Notes PDFmeetbalakumarОценок пока нет
- CATORCE Fats, OilsandWaxesДокумент11 страницCATORCE Fats, OilsandWaxesJaymee DelfinadoОценок пока нет
- Introduction:Composition of Petroleum, Laboratory Tests, Refinery Feedstocks and ProductsДокумент17 страницIntroduction:Composition of Petroleum, Laboratory Tests, Refinery Feedstocks and ProductsZaid YahyaОценок пока нет
- Petroleum RefineryДокумент3 страницыPetroleum RefinerySaba JavedОценок пока нет
- CHE 410 4 Petroleum ProductsДокумент23 страницыCHE 410 4 Petroleum ProductsEilyza AballaОценок пока нет
- WAX AddetivesДокумент16 страницWAX AddetivesAlbert GhobrialОценок пока нет
- Classification and Differentiation of IRAQI Crude Oils by Refractive IndexДокумент44 страницыClassification and Differentiation of IRAQI Crude Oils by Refractive Indexfarah al-sudaniОценок пока нет
- PetroleumДокумент8 страницPetroleumM AbdullahОценок пока нет
- Chemical Composition of PetroleumДокумент18 страницChemical Composition of PetroleumVeera Reddy BijjaramОценок пока нет
- 1-Processing of PetroleumДокумент28 страниц1-Processing of PetroleumNurfatihah UmairahОценок пока нет
- Hydraulic Oil DescriptionДокумент4 страницыHydraulic Oil DescriptionSeindahNyaОценок пока нет
- 4 FuelsДокумент54 страницы4 FuelsZemariam GetuОценок пока нет
- Liquid Fuels - NewДокумент24 страницыLiquid Fuels - NewChandrasekhar DevarapuОценок пока нет
- Solvents and DiluentsДокумент5 страницSolvents and DiluentsChris FordОценок пока нет
- Unit 4-Lecture 3-Liquid Fuel, Gaseous Fuel & Alternative FuelsДокумент24 страницыUnit 4-Lecture 3-Liquid Fuel, Gaseous Fuel & Alternative FuelsLadliОценок пока нет
- Petrolium P 2020Документ13 страницPetrolium P 2020dashrath singhОценок пока нет
- French Polishing and Enamelling A Practical Work of InstructionОт EverandFrench Polishing and Enamelling A Practical Work of InstructionОценок пока нет
- Sika PDS - E - Sikagard - 705 LДокумент3 страницыSika PDS - E - Sikagard - 705 Llwin_oo2435Оценок пока нет
- DRM Wha B PDFДокумент31 страницаDRM Wha B PDFBryan JohnsonОценок пока нет
- Handling of Gaseous Fuels: Caroline P. Mirandilla Catherine C. Glorioso Josua Royce S. RuzolДокумент16 страницHandling of Gaseous Fuels: Caroline P. Mirandilla Catherine C. Glorioso Josua Royce S. RuzolRonald Andrei DaguioОценок пока нет
- New Text DocumentДокумент6 страницNew Text DocumentsadsdОценок пока нет
- Remote Control Programming GuideДокумент47 страницRemote Control Programming GuideArslan Saleem0% (1)
- E SN752Документ3 страницыE SN752hasan_676489616Оценок пока нет
- Shaper, Slotter and PlanerДокумент9 страницShaper, Slotter and PlanerRenjith RajendraprasadОценок пока нет
- Spaulding Lighting Ventura Spec Sheet 8-84Документ2 страницыSpaulding Lighting Ventura Spec Sheet 8-84Alan MastersОценок пока нет
- Softening Point TestДокумент11 страницSoftening Point Testkadhim Ali81% (27)
- Global Talent 2021Документ48 страницGlobal Talent 2021Mentari Clara dewantiОценок пока нет
- Merit Safety SolutionsДокумент4 страницыMerit Safety SolutionsradeonunОценок пока нет
- KAESER SX6 Service ManualДокумент100 страницKAESER SX6 Service ManualYassin AlkadyОценок пока нет
- Montagehandbuch Geschl en FinalДокумент68 страницMontagehandbuch Geschl en FinalgamalОценок пока нет
- 88 m37Документ8 страниц88 m37Mohammed Essam ShatnawiОценок пока нет
- Hall Sensors Selection GuideДокумент2 страницыHall Sensors Selection GuideMiltongrimi GrimilОценок пока нет
- EAS 410-2005 - Dumu ZAS Mabati - decryptedKLRДокумент11 страницEAS 410-2005 - Dumu ZAS Mabati - decryptedKLRPEng. Tech. Alvince KoreroОценок пока нет
- Chemical Kinetics Mcqs Group 1Документ11 страницChemical Kinetics Mcqs Group 1zafarchem_iqbalОценок пока нет
- Dow Coring Asia ManualДокумент69 страницDow Coring Asia ManualGULJAR SINGHОценок пока нет
- BS 00489-1999 PDFДокумент10 страницBS 00489-1999 PDFNayan jainОценок пока нет
- Tipos Concavos y MantosДокумент22 страницыTipos Concavos y MantosJose Luis Atao SantiagoОценок пока нет
- Sample Leak Testing Report For Ast TankДокумент1 страницаSample Leak Testing Report For Ast TankArimoro Cyril Obuse43% (7)
- Chemical Composition, Mechanical, Physical and Environmental Properties of SAE 1536, Steel Grades, Carbon SteelДокумент1 страницаChemical Composition, Mechanical, Physical and Environmental Properties of SAE 1536, Steel Grades, Carbon SteelKuldeep SinghОценок пока нет
- Operation of The First HIsmelt Plant in ChinaДокумент8 страницOperation of The First HIsmelt Plant in ChinaJJОценок пока нет
- Simulation of Draping, Infiltration and Curing of CompositesДокумент49 страницSimulation of Draping, Infiltration and Curing of CompositesPeti Kovács100% (1)
- Tool/ Equipment Description/ Function Picture A. Measuring ToolsДокумент13 страницTool/ Equipment Description/ Function Picture A. Measuring ToolsNicolas AntiguaОценок пока нет
- Cad 4 1-4 35Документ12 страницCad 4 1-4 35mtdestaОценок пока нет
- Jurnal SanitasiДокумент12 страницJurnal SanitasiAnnisa FadlilahОценок пока нет
- Chap 1Документ22 страницыChap 1Zara Sikander33% (3)
- Artigo - Contribuição em Estudos Reológicos de Autonivelante Com Adição de Resíduo Rocha OrnamentalДокумент12 страницArtigo - Contribuição em Estudos Reológicos de Autonivelante Com Adição de Resíduo Rocha OrnamentalDjalma NetoОценок пока нет
- All Sites DPR 02-06-2017Документ16 страницAll Sites DPR 02-06-2017San SvsОценок пока нет