Академический Документы
Профессиональный Документы
Культура Документы
Chemical Reactions - Monitoring The Rate of Reaction Worksheet PDF
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Chemical Reactions - Monitoring The Rate of Reaction Worksheet PDF
Авторское право:
Доступные форматы
SCIENCE TEACHER
Chemical reactions
Monitoring the rate of reaction between a metal carbonate and a
dilute acid by recording loss of mass SW-CHE-ADV-0002
Objective
To measure the mass of the reaction mixture in a metal carbonate–acid reaction at regular
intervals and use the data collected to see how the rate of reaction changes over time.
Instructions
1. Measure 50 cm3 of dilute hydrochloric acid (1.0 mol dm–3) and pour it into a 250 cm3 conical
flask.
2. Weigh out between 1.20 and 1.30 g of calcium carbonate in a weighing dish.
3. Place the conical flask and the weighing dish on a top pan balance and record their
combined mass.
Dilute
hydrochloric
acid
Calcium
carbonate
4. Pour the calcium carbonate into the conical flask and start the stop watch.
5. Insert a plug of cotton wool in the mouth of the flask and replace the weighing dish on the
balance.
6. Record the mass of the reaction mixture and apparatus every half minute until the reaction
stops and no more bubbles of gas are released.
© Macmillan Publishers Ltd 2007
SCIENCE TEACHER
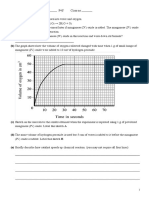
7 Write your results in the following table.
Time/min Mass/g Mass loss/g Time/min Mass/g Mass loss/g
0.0 0.0 5.0
0.5 5.5
1.0 6.0
1.5 6.5
2.0 7.0
2.5 7.5
3.0 8.0
3.5 8.5
4.0 9.0
4.5 9.5
8. Calculate the loss of mass each half minute.
9. Draw a graph of loss of mass against time.
Conclusions
Thinking about the following questions may help you to write your conclusions.
1. Is your graph a straight line or a curve?
2. What does the shape of the graph tell you about the rate at different times during the
reaction?
3. What was the total mass lost during the reaction?
4. After how many minutes was all of the calcium carbonate used up?
5. When was the rate of reaction greatest?
6. Can you suggest a reason why this was the case?
© Macmillan Publishers Ltd 2007
Вам также может понравиться
- Aces 2015041014584820 PDFДокумент5 страницAces 2015041014584820 PDFvtnhoemОценок пока нет
- Atoomus مﻮﺘﻌﻟا ﺪﻤﺣا ﻖﻴﻓﻮﺗ ﻦﻣﺆﻣДокумент6 страницAtoomus مﻮﺘﻌﻟا ﺪﻤﺣا ﻖﻴﻓﻮﺗ ﻦﻣﺆﻣahm531Оценок пока нет
- 14 Chapter 6Документ13 страниц14 Chapter 68925138229Оценок пока нет
- Exp 4-Noor Syaza Aqilah-2019317049-As2466a1Документ9 страницExp 4-Noor Syaza Aqilah-2019317049-As2466a1syazaqilah100% (1)
- Ex OxydissolvedДокумент3 страницыEx OxydissolvedrobertОценок пока нет
- HowfactorsaffectratesДокумент1 страницаHowfactorsaffectratesDOPPLER EFFECTОценок пока нет
- Lab#8Документ3 страницыLab#8myhbtggbhnjnОценок пока нет
- 2nd Sem Chemistry ManualДокумент19 страниц2nd Sem Chemistry ManualOliver Ryan FernandesОценок пока нет
- Science BookletДокумент10 страницScience BookletBEYZA POYRAZОценок пока нет
- Huang 2018 - HPLC - ESPECIFICAÇÕESДокумент10 страницHuang 2018 - HPLC - ESPECIFICAÇÕESPoliana PinheiroОценок пока нет
- Chem ProjectДокумент4 страницыChem ProjectsawОценок пока нет
- AP Chem Acids and Bases NameДокумент2 страницыAP Chem Acids and Bases NameKristine BaguinatОценок пока нет
- Experiment 5: The Determination of Relative Atomic Mass of MagnesiumДокумент2 страницыExperiment 5: The Determination of Relative Atomic Mass of MagnesiumJongFungОценок пока нет
- Chem 2 Weak Base Strong Acid Lab ReportДокумент6 страницChem 2 Weak Base Strong Acid Lab ReportMohammad Izadi100% (1)
- US4175066 Dispersante Carbonato Calcio - AA-AMALEICO PDFДокумент4 страницыUS4175066 Dispersante Carbonato Calcio - AA-AMALEICO PDFpasalacqua85Оценок пока нет
- Recent Advances in Copper Heap Leach Analysis and InterpretationДокумент26 страницRecent Advances in Copper Heap Leach Analysis and InterpretationChelseaОценок пока нет
- Practicum Report 1 (Stoichometry)Документ8 страницPracticum Report 1 (Stoichometry)Difa RahmatikaОценок пока нет
- Carbonate AcidizingДокумент30 страницCarbonate AcidizingJhonny E Col ArОценок пока нет
- Ease 4 Chemistry Grade 8 19-20Документ8 страницEase 4 Chemistry Grade 8 19-20fajarОценок пока нет
- Chem 26.1 ProbSet1 1stSemAY1920Документ3 страницыChem 26.1 ProbSet1 1stSemAY1920LoeyОценок пока нет
- New Approacg On Drilling Fluids Technology To Improve Drilling PerformanceДокумент10 страницNew Approacg On Drilling Fluids Technology To Improve Drilling PerformanceJonathan FariasОценок пока нет
- Experiment 6 & 7Документ10 страницExperiment 6 & 7gajenraoОценок пока нет
- HL PAPER 3 MockДокумент37 страницHL PAPER 3 MockIda Bagus Alit ManuabaОценок пока нет
- PH Buffers 3 QPДокумент9 страницPH Buffers 3 QPnayyarshria06Оценок пока нет
- Chemistry Report (Size of Reactant)Документ3 страницыChemistry Report (Size of Reactant)Ain NajwaОценок пока нет
- Lab Report 1Документ27 страницLab Report 1szulkipeliОценок пока нет
- CHAPTER 5. Durability of A Novel... - Sin Figuras (Revised)Документ43 страницыCHAPTER 5. Durability of A Novel... - Sin Figuras (Revised)Humbert Day-LewisОценок пока нет
- Experiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Документ15 страницExperiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Noor Nasuha Noor AriffinОценок пока нет
- Cement Stablied Updated FileДокумент11 страницCement Stablied Updated FileRitesh ChauhanОценок пока нет
- Lampiran A Contoh PerhitunganДокумент10 страницLampiran A Contoh PerhitunganIka Safitri RachmawatiОценок пока нет
- Stable of Calcium GluconateДокумент4 страницыStable of Calcium GluconateTaurusVõОценок пока нет
- Gabrielle Robinson - 601 Labs 2021Документ13 страницGabrielle Robinson - 601 Labs 2021Gabrielle RobinsonОценок пока нет
- Lab ReportДокумент48 страницLab ReportAthirah JeffryОценок пока нет
- J Egypro 2013 06 511Документ5 страницJ Egypro 2013 06 511LACHEHAB AdilОценок пока нет
- Lab Iodine ClockДокумент3 страницыLab Iodine ClocknamalОценок пока нет
- Esterification of EthanolДокумент15 страницEsterification of EthanolSadia HasanОценок пока нет
- Exer 2 Post-Lab ReportДокумент6 страницExer 2 Post-Lab ReportKin DemoticaОценок пока нет
- IPTC 12368 Optimizing Well Productivity by Controlling Acid Dissolution Pattern During Matrix Acidizing of Carbonate ReservoirsДокумент1 страницаIPTC 12368 Optimizing Well Productivity by Controlling Acid Dissolution Pattern During Matrix Acidizing of Carbonate ReservoirsJoseJavier ColinaОценок пока нет
- Effect of Surface Area On The Rate of ReactionДокумент10 страницEffect of Surface Area On The Rate of ReactionsuffyandaimОценок пока нет
- SCH4C Percentage YieldДокумент8 страницSCH4C Percentage YieldSteve M HallОценок пока нет
- Beyond BenignДокумент8 страницBeyond BenignVictor Akinseye OluwatoyinОценок пока нет
- Exercise On Rate of ReactioДокумент4 страницыExercise On Rate of ReactiohahaОценок пока нет
- Title: Rate of Reaction Hypothesis: The Rate of Reaction Will Depend On Concentration of Reactants Used. ObjectiveДокумент3 страницыTitle: Rate of Reaction Hypothesis: The Rate of Reaction Will Depend On Concentration of Reactants Used. ObjectivecyihueyОценок пока нет
- Identification of Ions and Gases: Question Paper 2Документ8 страницIdentification of Ions and Gases: Question Paper 2Shlok And gauransh legendОценок пока нет
- Activity 13.3: How Does Concentration Affect Reaction Rate?Документ2 страницыActivity 13.3: How Does Concentration Affect Reaction Rate?gkawsar22Оценок пока нет
- WINSEM2022-23 TCHY103P LO VL2022230506444 2023-03-01 Reference-Material-IДокумент3 страницыWINSEM2022-23 TCHY103P LO VL2022230506444 2023-03-01 Reference-Material-ISachinОценок пока нет
- Mazoe Floatation ProjectДокумент14 страницMazoe Floatation ProjectEDSON CHENJERAIОценок пока нет
- Chem 451 Physical Chemistry IIДокумент7 страницChem 451 Physical Chemistry IIjeamnard balitaanОценок пока нет
- Module 03 Solid Liquid ReactionДокумент4 страницыModule 03 Solid Liquid ReactionFarah -HОценок пока нет
- Chemical Engineering Laboratory III: Hardness Removal With Ion Exchange MethodДокумент7 страницChemical Engineering Laboratory III: Hardness Removal With Ion Exchange MethodVestel ÇallıОценок пока нет
- Che 3104 Ion Exchanger ExperimentДокумент15 страницChe 3104 Ion Exchanger Experimentapi-408316181Оценок пока нет
- Evaluación y Predicción de La Depositación de Carbonato de Calsio en SuperficiesДокумент14 страницEvaluación y Predicción de La Depositación de Carbonato de Calsio en SuperficiesWilmarAlexisRamirezОценок пока нет
- Ojic 2016042713213847 PDFДокумент10 страницOjic 2016042713213847 PDFJesha LibreaОценок пока нет
- 3 s2.0 B9780128187661003196 MainДокумент5 страниц3 s2.0 B9780128187661003196 MainJ MrОценок пока нет
- Catalase LabДокумент6 страницCatalase Laboofnivlak5100% (11)
- BiochemistryДокумент10 страницBiochemistryjohnny brooksОценок пока нет
- S.no. Name of The Experiment Date of Conduction Date of Submission P2 Cascade CSTR 4 February, 2021 9 February, 2021Документ14 страницS.no. Name of The Experiment Date of Conduction Date of Submission P2 Cascade CSTR 4 February, 2021 9 February, 2021DEEPSHIKA DUTTAОценок пока нет
- LabДокумент47 страницLabYuen Kim0% (1)
- HTR India - Products - Current Sense Resistors - Ceramic Encased Resistor - BR (English)Документ4 страницыHTR India - Products - Current Sense Resistors - Ceramic Encased Resistor - BR (English)crplzОценок пока нет
- Cytotoxic Screening of Tropical Plants Using Brine Shrimp Lethality TestДокумент20 страницCytotoxic Screening of Tropical Plants Using Brine Shrimp Lethality TestPrograma BRICОценок пока нет
- Liste Des FiltresДокумент6 страницListe Des FiltresYacine MokhtariОценок пока нет
- C0 2 Corrosion of Carbon Steel - From Mechanistic To Empirical ModellingДокумент30 страницC0 2 Corrosion of Carbon Steel - From Mechanistic To Empirical ModellingJannet GalvanОценок пока нет
- INTRO To ORGANIC CHEMISTRYДокумент60 страницINTRO To ORGANIC CHEMISTRYNailah KaharОценок пока нет
- Alfa Laval Heating and Cooling Hub Air-Conditioning and Chillers BrochureДокумент6 страницAlfa Laval Heating and Cooling Hub Air-Conditioning and Chillers BrochureEmmaОценок пока нет
- Hi9813 6 - Hi9813 5Документ4 страницыHi9813 6 - Hi9813 5Vani IIОценок пока нет
- Henley's 20th Century Home - Workshop FormulasДокумент848 страницHenley's 20th Century Home - Workshop FormulasEric MalainОценок пока нет
- Stereochemistry - HandoutДокумент10 страницStereochemistry - Handoutjoseph cyron solidumОценок пока нет
- Lead Acid vs. Lithium-Ion Battery ComparisonДокумент5 страницLead Acid vs. Lithium-Ion Battery ComparisonRasbihari SharmaОценок пока нет
- Addition of Ref#2080, #2081, #2082, #2083 and #2084. D D: EB-455Wi/465i/450W/450Wi/460/460iДокумент15 страницAddition of Ref#2080, #2081, #2082, #2083 and #2084. D D: EB-455Wi/465i/450W/450Wi/460/460ifefotroncitoОценок пока нет
- 3d Woven Composite FatigueДокумент11 страниц3d Woven Composite FatigueSri SaiОценок пока нет
- Modelling Discontinuous Rock With FLAC and UDECДокумент50 страницModelling Discontinuous Rock With FLAC and UDECAbhishek P SaiОценок пока нет
- SN1 V SN2 (Nucleophilic Substitution Again) (A2)Документ3 страницыSN1 V SN2 (Nucleophilic Substitution Again) (A2)Kevin The Chemistry TutorОценок пока нет
- 40 Item Test Science 6with Key To CorrectionДокумент5 страниц40 Item Test Science 6with Key To CorrectionvinnОценок пока нет
- Niels Bohr Atomic Theory BohrДокумент1 страницаNiels Bohr Atomic Theory BohrAyessa AnchetaОценок пока нет
- GCC SyallbusДокумент12 страницGCC SyallbusAbheek KashyapОценок пока нет
- 03 FIRE BOOST Aftertreatment UsersGuideДокумент318 страниц03 FIRE BOOST Aftertreatment UsersGuidehenevil0% (1)
- Block Diagram: ExplanationДокумент26 страницBlock Diagram: Explanationmr ozairОценок пока нет
- Planck Constant - Wikipedia, The Free EncyclopediaДокумент15 страницPlanck Constant - Wikipedia, The Free Encyclopediad_richard_dОценок пока нет
- Gold Extraction With Halogens: J.-M. Lalancette, B. Dubreuil, D. Lemieux and C. ChouinardДокумент16 страницGold Extraction With Halogens: J.-M. Lalancette, B. Dubreuil, D. Lemieux and C. ChouinardLudwig Kommer100% (2)
- Type 2Документ7 страницType 2AnOnYmOuS_1995Оценок пока нет
- APIДокумент4 страницыAPIAnam Hyat KhanОценок пока нет
- Activated Carbon From Jackfruit Peel Waste by H3POДокумент13 страницActivated Carbon From Jackfruit Peel Waste by H3POMaria De La HozОценок пока нет
- Supervision Manual As Guidance For Supervisory StaffДокумент20 страницSupervision Manual As Guidance For Supervisory StaffAxelОценок пока нет
- DT Series Digital TachometerДокумент3 страницыDT Series Digital TachometerMamani JesusОценок пока нет
- 100M ReaxДокумент1 страница100M ReaxNagy AliОценок пока нет
- AN 280 IC Carbohydrates Coffee HPAE PAD AN70231 ENДокумент12 страницAN 280 IC Carbohydrates Coffee HPAE PAD AN70231 ENjoann bОценок пока нет
- Thesis PagesДокумент68 страницThesis PagesarunОценок пока нет