Академический Документы
Профессиональный Документы
Культура Документы
Ternary Phase Diagram at 30deg
Загружено:
Alistair GellanИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Ternary Phase Diagram at 30deg
Загружено:
Alistair GellanАвторское право:
Доступные форматы
NOMENCLATURE Hering, H., Perio, P., Bull. Soc. Chim. France 1952, p. 351.
Ibbs, T.L., Underwood, L., Proc. Roy. SOC.(London) 39,
a = thermodynamic activity 234 (1926).
K = equilibrium constant Kubaschewski, O., Evans, E.L., "Metallurgical Thermo-
N = mole fraction chemistry," Wiley, New York, 1956.
Q = independently measured variable Mallet, M.W., Gerds, A.F., Vaughn, D.A., J . Electro Chem.
P = pressure, atm. SOC.98, 505 (1951).
Q = ith measured independent variable Rundle, R.A., Baenziger, X.C., Wilson, A.S., McDonald,
R = probable error in Q R.A., J . Am. Chem. SOC.70, 99 (1948).
r, = probable error in q cmeasurement Scarborough, J.B., "Numerical Mathematical Analysis," 4th
T = temperature, Kelvin ed., Johns Hopkins Press, Baltimore, 1950.
u ' = weight factor Vaughn, D.A., Melton, C.W., Gerds, A.F., Battelle Memorial
A@ = Gibbs free energy change, cal. /gram mole Inst. Rept. No. 1175, 1957.
B = Bragg diffraction angle Williams, J., Sambell, R.A.J., J . Less Common Metals 1,
d J = complement of Bragg diffraction angle 217 (1959).
Williams, J., Sambell, R.A.J., Wilkinson, D., U . K . At.
Energy Authority Res. Group Memo. (AERE-M), No. 625,
LITE RAT URE CITED 1960.
Wilson, W.B., J . Am. Ceram. SOC.43, 77 (1960).
(1) Austin, A.E., Gerds, A.F., Battelle Memorial Inst. Rept.
No. 1272, 1958. RECEIVEDfor review March 1, 1962. Accepted July 5, 1962.
(2) Beers, Y., "Introduction to the Theory of Error," Addison Work supported with assistance of a Michigan Memorial Phoenix
Wesley Publ., Reading, Mass., 1957. Project Grant. Based in part on a thesis by J.R. Piazza, submitted
(3) Coughlin, J.P., U.S. Bur. of Mines Bull. No. 542, 1954. to the Horace H. Rakham School of Graduate Studies of the
(4) Grieveson, P., Ph. D. thesis, University of London, 1960. University of Michigan for part of the Ph. D. degree in metallurgical
(5) Gronvold, F., J . Inorg. Nuclear Chem. 1,357 (1955). engineering.
Solubility and Equilibrium Data of Phenol-Water-Isoamyl Acetate
and Phenol-Water-Methyl Isobutyl Ketone Systems at 30' C.
K. S. NARASIMHAN, C. C. REDDY, and K. S. CHAR1
Regional Research Laboratory, Hyderabad 9, India
S O L U B I L I T Y and equilibrium data for the system
Solvents Used
phenol-water-n-butyl acetate (system 1) a t 30" C. were
reported in a previous communication ( 4 ) . T h e present Purified Lit.
article deals with the systems phenol-water-isoamyl Sample Value Ref.
acetate and phenol-water-methyl isobutyl ketone a t Isoamyl acetate
30" C. As in the previous system, the solubility data
of the ternary systems were determined first, followed by Ester content, 5 100 ... ...
Specific gravity, 24'4" C. 0.8670 0.8664 (3)
the determination of the equilibrium data. Refractive index. 20" C. 1.4005 1.4005 (3)
Methyl isobutyl ketone
MATE RIALS
Ketone content, 5 99.1-99.5 ... ...
Phenol. Phenol was distilled in a glass Quick-fit distilla- Specific gravity. 2 0 1 2 0 C.
~ 0.7973 0.8042 (3)
tion apparatus. The fraction obtained a t the boiling point Refractive index, 20" C. 1.3960 1.3958 (3)
corresponding to 181.7" C. a t 760 mm. having a purity
of 99.935 was used. EXPERIMENTAL PROCEDURE
Water. Pure distilled water was used.
Isoamyl Acetate. Isoamyl acetate was fractionated twice Solubility data were obtained by the turbidity end point
in Tower's fractionating column and the fraction within method as described by Othmer, White, and Trueger (6).
2" C , of the boiling point corresponding to 140.1 - 42.1" C. The experimental details were similar t o those of the
a t 760 mm. was used. earlier system ( 4 ) . Tables I and I1 give the solubility data
Methyl Isobutyl Ketone (MIBK). Commercial methyl iso- in weight fractions for systems phenol-water-isoamyl
butyl ketone was similarly fractionated and the fraction acetate (system 2 ) and phenol-water-methyl isobutyl
corresponding to 113.9 - 15.5" C. a t 760 mm. was used. ketone (system 3), respectively. Mutual solubilities of
VOL. 7, No. 4, OCTOBER 1962 457
Table I. Solubility Data for the System Phenol-Water- Table (I. Solubility Data for the System Phenol-Water-Methyl
Isoamyl Acetate at 30" C. (Weight fractions) Isobutyl Ketone (MIBK) at 30" C. (Weight fractions)
Isoamyl Phenol Water MIBK R.I.
Phenol Water Acetate R.I. Solvent-RichPhase
Solvent-RichPhase 0.0146 0.9854 1.3912
0.0058 0.9942 1.3960 o.is49 0.0424 0.6727 1.4315
0.1248 0.0136 0.8616 1.4425 0.3318 0.0486 0.6196 1.4381
0.1969 0.0232 0.7799 1.4225 0.3538 0.0493 0.5969 1.4415
0.2622 0.0234 0.7144 1.4320 0.4059 0.0506 0.5435 1.4490
0.3265 0.0308 0.6427 1.4399 0.4460 0.0563 0.4977 1.4550
0.4311 0.0413 0.5276 1.4550 0.5050 0.0670 0.4280 1.4630
0.4649 0.0497 0.4854 1.4600 0.5610 0.0742 0.3648 1.4710
0.4968 0.0564 0.4468 1.4640 0.5898 0.0800 0.3302 1.4748
0.5325 0.0637 0.4038 1.4690 0.6383 0.0952 0.2665 1.4825
~ . ~ .
0.5702 0.0713 0.3585 1.4745 0.6816 0.1183 0.2001 1.4865
0.5772 0.0812 0.3416 1.4755 0.7000 0.1399 0.1601 1.4880
0.6469 0.1062 0.2469 1.4829 0.7386 0.1789 0.0825 1.4918
0.7025 0.1479 0.1496 1.4882 0.7256 0.2459 0.0285 1.4862
0.7221 0.2095 0.0684 1.4862 0.6935 0.3065 ... 1.4779
0.6960 0.3040 ... 1.4779 Water-Rich Phase
Water-Rich Phase 0.9913 0.0087 1.3335
0.9978 0.0022 1.3325 0.oii3 0.9814 0.0063 1.3352
o.oii2 0.9874 0.0014 1.3340 0.0206 0.9753 0.0041 1.3370
0.0217 0.9773 0.0010 1.3360 0.0290 0.9681 0.0029 1.3385
0.0271 0.9720 0.0009 1.3372 0.0374 0.9608 0.0018 1.3400
0.0423 0.9573 0.0004 1.3405 0.0526 0.9465 0.0009 1.3430
0.0608 0.9389 0.0003 1.3442 0.0856 0.9144 ... 1.3490
0.0880 0.9120 ... 1.3489
Table Ill. Mutual Solubilities at 30" C. as Weight Fractions
water and isoamyl acetate, and water and MIBK, with the Snlv
literature values are compared in Table 111. Mutual Lit.
solubilities between water and isoamyl acetate show close Observed value Ref.
agreement between the observed and literature values; but Isoamyl acetate in water 0.0022 0.0020 (8)
the solubility of MIBK in water appears to be lower than Water in isoamyl acetate 0.0058 0.0040 (8)
the reported values ( 2 ) . Tables I and I1 also give the Methyl isobutyl
refractive indices (for the sodium D line) for the ternary ketone in water 0.0087 0.0166 (2)
saturated mixtures. These were used later for finding the Water in methyl
equilibrium data in the analysis of ternary saturated isobutyl ketone 0.0146 ... ...
mixtures.
for phenol concentration by Koppeschar's method (7) as
EQUlLI BRI UM DATA
a check on the refractive index method. The equilibrium
The data were determined by the method reported data (in weight fractions) for the two systems are reported
earlier ( 4 ) . The phenol-water-solvent mixtures were well in Tables IV and V and are represented in Figures 1 and 2.
stirred in the apparatus a t 30" C. and allowed to settle.
The refractive indices of two phases which were in equili- OBSERVATIONS
brium with each other were determined. The compositions
of the ternary mixtures were obtained with the help of the The phase behavior of these two systems is similar to
plots drawn previously connecting refractive index and the that of the phenol-water-butyl acetate system, in which
composition of ternary saturated mixtures at 30" C. two pairs are partially miscible. Binodal curves for all
Samples of each layer in equilibrium were also analyzed three systems are compared in Figures 3 (solvent-rich
PUEWL PHENOL
Figure 1. Equilibrium data for the system Figure 2. Equilibrium
phenol-water-amyl acetate data for the system
I phenol-water-MIBK
I f L 3 4 8 6 7 8 , l e 3 4 6 6 7 0 0
WATER A W L AOCTATL WATER METHYL ISOBUTYL KETONE
458 JOURNAL OF CHEMICAL AND ENGINEERING DATA
Table V. Equilibrium Data for the System Phenol-Water-Methyl
Table IV. Equilibrium Data for the System Phenol-Water-
Isobutyl Ketone (MIBK) at 30" C. (Weight fractions)
Isoamyl Acetate a t 30" C. (Weight fractions)
Phenol Water MIBK R.I.
Isoamyl Extract Phase
Phenol Water Acetate R.I. 0.0146 0.9854 1.3912
Extract Phase o.ii0o 0.0350 0.8250 1.4115
0.1725 0.0390 0.7885 1.4165
... 0.0058 0.9942 1.3960 0.2150 0.0420 0.7430 1.4222
0.0875 0.0125 0.9000 1.4080 0.2425 0.0430 0.7145 1.4260
0.1675 0.0225 0.8100 1.4190 0.2650 0.0440 o.min
..~. 1.4290
- ._..
0.2300 0.0275 0.7425 1.4275 0.3325 0.0480 0.6195 1.4388
0.3200 0.0375 0.6425 1.4395
~ ~ ~ . . 0.3800 0.0520 0.5680 1.4456
0.4150 0.0450 0.5400 1.4525 0.4150 0.0540 0.5310 1.4505
0.4900 0.0550 0.4550 1.4625 0.4675 0.0590 0.4735 1.4580
0.5650 0.0700 0.3650 1.4728 0.5250 0.0660 0.4090 1.4659
0.6600 0.1100 0.2300 1.4845 0.5800 0.0770 0.3430 1.4735
0.6800 0.1325 0.1875 1.4865 0.6225 0.0890 0.2885 1.4790
0.7175 0.1875 0.0950 1.4872 0.6450 0.0980 0.2570 1.4822
0.6960 0.3040 ... 1.4779 0.7075 0.1410 0.1515 1.4889
0.7300 0.2320 0.0380 1.4885
0.7175 0.2620 0.0165 1.4839
Raffinate Phase 0.6936 0.3065 ... 1.4779
0.9978 0.0022 1.3325 Raffinate Phase
0.0025 0.9955 0.0020 1.3328 0.9913 0.0087 1.3335
0.0050 0.9932 0.0018 1.3331 0.0035 0.9880 0.0085 1.3339
0.0085 0.9899 0.0016 1.3335 0.0045 0.9875 0.0080 1.3340
0.0165 0.9822 0.0013 1.3350 0.0052 0.9870 0.0078 1.3341
0.0252 0.9739 0.0009 1.3368
~ ~~~~
0.0060 0.9865 0.0075 1.3342
0.0308 0.9684 0.0008 1.3380 0.0067 0.9860 0.0073 1.3343
0.0425 0.9570 0.0005 1.3405 0.0080 0.9845 0.0075 1.3345
0.0570 0.9426 0.0004 1.3435 0.0112 0.9825 0.0063 1.3350
0.0595 0.9402 0.0003 1.3440 0.0140 0.9802 0.0058 1.3355
0.0685 0.9313 0.0002 1.3455 0.0187 0.9767 0.0046 1.3365
0.0880 0.9120 ... 1.3489 0.0237 0.9725 0.0038 1.3375
0.0315 0.9655 0.0030 1.3390
0.0370 0.9610 0.0020 1.3400
0.0422 0.9560 0.0018 1.3410
phase) and 4 (water rich phase). A tangent drawn from the 0.0555 0.9440 0.0005 1.3435
solvent point to the binodal curve, shows that the maximum 0.0690 0.9307 0.0003 1.3460
0.0790 0.9209 0.0001 1.3475
concentration to which dilute phenol solution can be 0.0856 0.9144 ... 1.3490
enriched using amyl acetate as solvent is over 91%; using
MIBK, it is 8970 a t 30" C. (Table VI).
During determination of the equilibrium data for the Table VI. Comparison of Three Solvents
system phenol-water-butyl acetate, it was observed for Enrichment
Soly., Wt. 7
phenol concentration between 59.25 and 65.05% in the of Phenol
extract phase that the densities of the two phases were in of Concn.,
very near to each other. A similar observation was made Solvent B.P., C. water water in, Wt. ?
in the present data. The densities of the two phases were Methyl isobutyl
found to be almost equal in the range 56.5 to 66.0% for ketone 115.9 0.87 1.46 89.0
the system phenol-water-isoamyl acetate and 64.5 to 70.8% n-Butyl acetate 126.0 0.29 1.45 91.0
for the system phenol-water-MIBK. Isoamyl acetate 142.1 0.22 0.58 91.5
PHENOL
PHENOL
.( BUTYL ACETATE
* BUTYL ACETATE AMYL ICETATE
AMYL ACETATE METHYL ISOBUTYL
KETONE
OMETHYL ISOBUTYL
,94 .os
I E 3 + 5 S 7 8 9 U .03 .04 .os ' 07 Q4
WATER SOLVENT WATER SOLVENT
Figure 3. Binodal curves for the systems Figure 4. Binodal curves for the systems in
in solvent rich phase waler rich phase
VOL. 7, No. 4, OCTOBER 1962 459
- SYSTEM I
t
-
P
Q
9.0 -
0 SYSTEM 3
1.0 -
Figure 6. Modified Dryden’s
correlation 0.2 0.4 0.6 0-0 1.0
/-- -P
92 0 4 0-6 0.8 I.0
-X
Figure 5. Bachman’s correlation for the systems 0.8
CORRE LATlON
The present data were analyzed by a number of methods
to check their consistency and to ascertain the methods
for their interpolation. The correlation suggested by
Othmer and Tobias ( 5 )
t
P
06
0.4
Log-
1-x = c, Log 1- Y
+ C?
X
~
Y
0.2
did not yield a straight line for the present data. but
correlation suggested by Bachman ( I ) did.
x= c3-
X -I c,
Y 0.2 0.4 0.6 0.8
Figure 5 gives Bachman’s correlation for all three systems.
Q-
The data for all the three systems could be represented Figure 7. Equilibrium relationships for
by a single straight line. the three systems on water-free basis
The modified Dryden’s relatidn
P NOMENCLATURE
- = CsP+Cs (3)
Q X = mole fraction of solvent (isoamyl acetate or MIBK)
in the extract phase
which was successfully used to correlate the data for the Y = mole fraction of residue (water) in the raffinate phase
system phenol-water-butyl acetate was applied to the P = mole fraction of solute (phenol) in the raffinate on
present system (Figure 6). Each system has yielded a water free basis
separate straight line. Q = mole fraction of solute (phenol) in the extract on
Figure 7 represents a plot of P us. Q for the three systems, water free basis
which is useful for the interpolation of data and theoretical C, to Cs = constants
stage calculations.
LITERATURE CITED
CONCLUSIONS (1) Bachman, Irvin, Id.Eng. Chem., Anal. E d . 12, 38 (1940).
n-Butyl acetate and isoamyl acetate give slightly better (2) Gross, P.M., Rientelin, J.C., Soylor, T.H., J . Phys. Chem. 43,
197 (1939).
enrichment for phenol than MIBK, and are less soluble (3) Marsden, C., “Solvents Manual,” p. 62, Cleaver Hume Press,
in water. London, 1954.
Bachman’s method as well as the modified Dryden’s (4) Narasimhan, K.S., Reddy, C.C., Chari, K.S., J. CHEM.ENG.
method appear to be useful for interpolation of the data DATA7, No. 3, 340 (1962).
for systems studied. (5) Othmer, D.F., Tobias, P.E., I n d . Eng. Chem. 34, 693 (1942).
(6) Othmer, D.F., White, R.E., Trueger, Edward, I n d . E n g . Chem.
33, 1240 (1941).
ACKNOWLEDGMENT (7) Redman, L.V., Weith, A.J., Brqck, F.P., Ibid., 5 , 389 (1913).
The authors express their sincere thanks to S. Husain (8) Venkatarathnam, A., Jagannatha Rao, R., Venkata Rao, C.,
Chem. Eng. Sci. 7, 102 (1957).
Zaheer, Director, Regional Research Laboratory, for his
keen interest in the work. RECEIVEDfor review April 19, 1962. Accepted June 25, 1962.
460 JOURNAL OF CHEMICAL AND ENGINEERING DATA
Вам также может понравиться
- Morse Potential NumberedДокумент5 страницMorse Potential NumberedAlistair GellanОценок пока нет
- Morse Potential NumberedДокумент5 страницMorse Potential NumberedAlistair GellanОценок пока нет
- Lol Bestest Spam EvarДокумент1 страницаLol Bestest Spam EvarAlistair GellanОценок пока нет
- AzeotropeReport Acetone 1atmДокумент1 страницаAzeotropeReport Acetone 1atmAlistair GellanОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Dynamic High School: Form Iii Terminal Exam - August 2021 PhysicsДокумент6 страницDynamic High School: Form Iii Terminal Exam - August 2021 PhysicsJoshuaОценок пока нет
- I0qm BalunДокумент2 страницыI0qm BalunDundo SakićОценок пока нет
- CFD Analysis of Liquid-Liquid Extraction Pulsed ColumnДокумент6 страницCFD Analysis of Liquid-Liquid Extraction Pulsed ColumnArunОценок пока нет
- Design, Analysis and Fabrication of Eddy Current Braking SystemДокумент11 страницDesign, Analysis and Fabrication of Eddy Current Braking SystemTEJAS V AОценок пока нет
- Laporan Kerja Ptaktik Pabrik Gula BungamayangДокумент81 страницаLaporan Kerja Ptaktik Pabrik Gula BungamayangArdiSaputraОценок пока нет
- Assing 4Документ2 страницыAssing 4KazaValiShaikОценок пока нет
- Force Summative Test Science 8Документ20 страницForce Summative Test Science 8lie villoteОценок пока нет
- smf28 PDFДокумент4 страницыsmf28 PDFNahed Ep KhaledОценок пока нет
- Energized Body - Superpower Wiki - FandomДокумент12 страницEnergized Body - Superpower Wiki - Fandomalex masonОценок пока нет
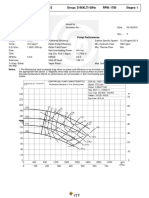
- Curva Goulds 3196Документ2 страницыCurva Goulds 3196edwinsazzz75% (4)
- IUT 2019 SolveДокумент15 страницIUT 2019 SolveAbdullah Al MahmudОценок пока нет
- Must Know-Physics (NMAT)Документ18 страницMust Know-Physics (NMAT)iya100% (2)
- Self Assessment MCQs - Students1Документ2 страницыSelf Assessment MCQs - Students1JunaidKhanОценок пока нет
- HydraulicsДокумент234 страницыHydraulicsChristine CastroОценок пока нет
- SCIENCEДокумент4 страницыSCIENCEMaria Dhalia MarquezОценок пока нет
- Engineering Mechanics NotesДокумент100 страницEngineering Mechanics NotesRagothsingh Ramadoss67% (3)
- The First Century of Chemical EngineeringДокумент6 страницThe First Century of Chemical EngineeringFredericoОценок пока нет
- Equilibrium of Floating BodiesДокумент18 страницEquilibrium of Floating BodiesMuneeb Rehman100% (1)
- Formulas and Reference Chart EocДокумент3 страницыFormulas and Reference Chart Eocapi-87739323Оценок пока нет
- An Introduction To The Kinetic Theory of Gases (Cambridge Science Classics) PDFДокумент321 страницаAn Introduction To The Kinetic Theory of Gases (Cambridge Science Classics) PDFShouvik MitraОценок пока нет
- Design Precast Boundary WallДокумент70 страницDesign Precast Boundary WallGajendra Bisht100% (2)
- 107D Iv Feg 05a 00012Документ68 страниц107D Iv Feg 05a 00012vinayak jadhavОценок пока нет
- Chemical Engineering ThermodynamicsДокумент35 страницChemical Engineering ThermodynamicsErik WeeksОценок пока нет
- Lab Mannual-Stewart and Gee's-2Документ17 страницLab Mannual-Stewart and Gee's-2Sitaramaraju VengalarajuОценок пока нет
- Modern Physics SampleДокумент10 страницModern Physics SampleRohit KumarОценок пока нет
- Meteodyn Complex Terrain Modeling CFD Software Bolund Hill Round Robin TestДокумент18 страницMeteodyn Complex Terrain Modeling CFD Software Bolund Hill Round Robin TestMeteodyn_UrbawindОценок пока нет
- Karmaveer Bhaurao Patil College Vashi, Navi Mumbai Autonomous CollegeДокумент21 страницаKarmaveer Bhaurao Patil College Vashi, Navi Mumbai Autonomous Collegemathers maddyОценок пока нет
- AAA - Stability - Lecture - SummaryДокумент146 страницAAA - Stability - Lecture - SummaryIvan EstradaОценок пока нет
- Let Table of Specification 2023-2024Документ13 страницLet Table of Specification 2023-2024COED ESSU100% (3)
- Column DesignДокумент4 страницыColumn DesignArnel Dodong83% (6)