Академический Документы
Профессиональный Документы
Культура Документы
US3136818-Produccion de Anilina
Загружено:
Joel Flores Castillo0 оценок0% нашли этот документ полезным (0 голосов)
38 просмотров4 страницыpatente US
Авторское право
© © All Rights Reserved
Доступные форматы
PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документpatente US
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
38 просмотров4 страницыUS3136818-Produccion de Anilina
Загружено:
Joel Flores Castillopatente US
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 4
3,136,818
United States Patent Office Patented June 9, 1964
2
3,136,818 is effected by leading a mixture of hydrogen and nitro
PRODUCTION OF ANLANE benzene over the catalyst present in the fluidized bed at
Heinrich Sperber and Guenter Poehler, Ludwigshafen the reaction temperature. The molar ratio of hydrogen to
(Rhine), Hans Joachim Pistor, Mannheim, and Anton
Wegerich, Limburgerhof, Germany, assignors to
nitrobenzene should be at least 3:1. We prefer a molar
excess of hydrogen of at least 20%, advantageously 50
Badische Anilin- & Soda-Fabrik Aktiengesellschaft, 400%, especially 50-250%. The nitrobenzene is intro
Lidwigshafen (Rhine), Germany duced in liquid or partly vaporized form together with
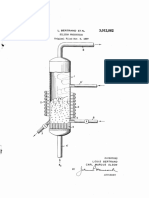
No Drawing. Filled Jan. 10, 1961, Ser. No. 8,678 the hydrogen. In accordance with the invention, the initial
Claims priority, application Germany Jan. 15, 1960 material is introduced into the fluidized layer at a plurality
4 Claims. (C. 260-580) 10 of points at different heights of the layer. Depending on
This invention relates to improvements in a process for the size of the reaction chamber and the height of the
the production of aniline by catalytic hydrogenation of fluidized layer, the initial material may be introduced at
nitrobenzene in a fluidized layer. two, three, four or more different heights, and introduc
It is known to produce aniline by catalytic hydrogena tion may be effected, for example, through nozzles. Nitro
tion of nitrobenzene. Since the reaction proceeds strongly 5 benzene and hydrogen are preferably introduced through
exothermically, considerable difficulty is experienced in the nozzles at a molar ratio of from 1:1 to 1:3.1. The
maintaining a uniform reaction temperature in the reac distance between inlets measured vertically should be at
tion chamber. Attempts have been made to overcome least 0.3 meter, especially 0.5 to 1.0 meter. In the case
the said difficulty by using a large excess of hydrogen in of very high fluidized layers, the distance may be several
order to lead away the heat. This measure, however, does 20 meters. If the diameter of a reaction space is compara
not prevent resinification on the catalyst and consequently tively large, a plurality of inlets is provided at the same
the activity of the catalyst subsides in a short time. Cool height. They are so arranged that their distance is at
ing means arranged within the catalyst chamber have least 0.3 meter from one another. It is convenient to dis
diminished this disadvantage without obviating it. It has tribute the inlets over the lower three-quarters of the
also been proposed to carry out the reaction in a fluidized 25 fluidized bed. It is advantageous to use for the introduc
layer. In this way the throughput can be increased, the tion, two-component nozzles which permit the simultane
nitrobenzene introduced in the liquid state and the regen ous introduction of nitrobenzene and hydrogen or hydro
eration period of the catalyst shortened, but on the other gen-containing gas. The fluidized condition is maintained
hand the rapid fall in activity of the catalyst cannot be by means of gas introduced from below. It is possible to
avoided solely by the use of a fluidized layer because when 30 use inert gases, as for example nitrogen, but it is preferable
liquid nitrobenzene is introduced at the lower end of the to use for this purpose the waste gases which still contain
vessel, the temperature in the vessel falls by reason of hydrogen, after separating water, and part of the hydrogen
rapid vaporization of the initial material and then con necessary for the reaction. It is also possible, however,
siderable evolution of heat occurs as a result of the exo to introduce part of the nitrobenzene with the flowing
thermic reaction, and gradual resinification. 35 gases from below into the fluidized bed. Or the whole
It is an object of the present invention to provide a amount of hydrogen can be introduced from below and
process for the catalytic hydrogenation of nitrobenzene the nitrobenzene alone introduced into the fluidized layer
to aniline in which the use of a large excess of hydrogen at the different heights.
is unnecessary. Another object of the invention is a The velocity of the gases in the fluidized layer lies be
process for the catalytic hydrogenation of nitrobenzene 40 tween 0.1 and 1.5 meters per second, especially 0.2 to 1.0
to aniline in which resinification of the catalyst is substan meter per second. The preferred velocity depends on the
tially precluded and consequently a prolonged life of the capacity of the catalyst for being fluidized. It is advan
catalyst is brought about. tageous to use globules or grains having a diameter of
These and other objects are achieved by carrying out about 0.05 to 0.5 mm., especially 0.15 to 0.4 mm.
the catalytic reaction of nitrobenzene with hydrogen to 45 The process may be carried out under normal pressure,
form aniline in a vessel which is provided with cooling but it is advantageous to apply increased pressure. Pres
means and in which the catalyst forms a fluidized layer, Sures of more than 4, especially 5 to 10 or more, e.g.,
at normal or increased pressure, and introducing the initial 10 to 25 atmospheres, are preferred. Higher pressures,
material to be hydrogenated at a plurality of places at for example 100 to 300 atmospheres, may also be used.
different heights of the vessel and introducing the hydro 50 When working under pressure and with loads in excess of
gen necessary for the hydrogenation together with the 0.5 kg. per liter of catalyst per hour, velocities between
initial material and/or at the bottom of the vessel. 20 and 80 cm. per second are sufficient. Moreover, work
Carrying out the catalytic hydrogenation of nitro ing under pressure prolongs catalyst life. When working
benzene in a fluidized layer by the method according to under pressure and with high loads, it is advantageous to
this invention leads to a practically complete reaction of 55 use a sufficient molar excess of hydrogen, for example of
the nitrobenzene with a very long life of the catalyst be 100-250 in order to avoid strong variations in the flow
fore it needs regeneration, and a very long total life. The speed within the reaction chamber.
life of the catalyst until it needs regeneration is that time Suitable catalysts include heavy metals of groups I, V,
which elapses before regeneration is necessary, taking into VI and VII of the periodic system as well as of the iron
account the amount of nitrobenzene reacted during this 60 and platinum group, for example copper, tungsten, mo
period. The total life of the catalyst is that time which lybdenum, nickel, cobalt or mixtures of these elements,
results from the sum of all the lives of the catalyst until as well as their oxides, sulfides or halides, possibly to
it needs regeneration, i.e., the total time for which the gether with boron or boron compounds. Examples of suit
able mixtures are cobalt molybdate and nickel wolframite.
catalyst can be used until regeneration can no longer be 65 They
successfully carried out. In carrying out the process in may be applied to carriers, such as alumina, natural
dustrially, both the life until regeneration is necessary and or synthetic silicates of aluminum, magnesium, zinc, iron,
the total life are important. The period until regenera
manganese, or titanium, pumice, iron oxide, magnesia, zinc
tion is necessary determines the frequency of regenera
oxide, zirconium oxide, titanium oxide, throium oxide or
mixtures thereof. The carriers may be treated with bro
tion. The total life is important for the frequency of 70 mine, iodine, fluorine or chlorine. It is preferred, espe
catalyst replacement. cially when carrying out the process under pressure, to
The catalytic hydrogenation of nitrobenzene to aniline use catalysts applied to silicic acid or silicate carriers
3,136,818
s 4.
which have a surface of more than 40 square meters per is used having a diameter of 1.2 meters and a height of 8
gram and a pore diameter of more than 20 A., and also meters in the lower end of which a grate is provided to
catalysts on alumina carriers which have an average pore ensure uniform distribution of the circulating gas supplied
diameter between 20 and 1000 A. The production of these to the vessel from below. A cyclone is provided in the
catalysts is known and is not a feature of the present in 5 upper part of the vessel to separate entrained catalyst. The
vention. reaction vessel contains 4 cubic meters of catalyst. The
The catalyst is reduced with hydrogen at about 200 C. catalyst consists of particles having a diameter of 0.2 to
at the beginning of the process. The catalyst remains in 0.3 mm. and has the composition 15% copper, 0.3%
the vessel and is regnerated by heating in a stream of air chromium, 0.3% barium and 0.3% zinc which are present
after a long period, for example after some weeks. How O as oxides and are applied to precipitated silicic acid, heated
ever, it is also possible to withdraw part of the catalyst to 400 C., as carrier. The vessel has twelve two-com
continuously or periodically and to replace it by fresh and/ ponent nozzles arranged in groups of three at different
or regenerated catalyst so that the process can be carried heights of the vessel. The first group are situated immedi
out continuously. ately above the grate and the second to fourth groups are
The gas can be passed through the vessel at a high 70, 140 and 210 cms. above the grate, i.e., at one-fifth,
velocity so that part of the catalyst is always withdrawn two-fifths and three-fifths of the height of the quiescent
and can be cooled and returned. Part of this amount of catalyst bed.
catalyst can be replaced by fresh or regenerated catalyst At the beginning, the catalyst is reduced, the air first
prior to its return. being driven out from the reaction vessel by introducing
The reaction temperature is kept between 200 and 400 nitrogen and then the catalyst being reduced with hy
C., preferably between 250 and 300° C. A tube nest drogen at a temperature between 200 and 250° C. After
containing a heat-absorbing liquid is provided in the vessel reduction of the catalyst, the working pressure in the
for heat withdrawal. For example steam may be pro vessel is adjusted to 5 atmospheres gage and then the
duced in the tubes in this way. The vessel walls may injection of liquid nitrobenzene through the two-com
similarly be provided with a cooling jacket, ponent nozzles in finely divided form is begun with the
The throughput of nitrobenzene is from 0.1 to 10, espe aid of the amount of hydrogen necessary for the reduc
cially 0.2 to 3, kilograms per liter of catalyst volume per tion, namely 3.1 mois per mol of nitrobenzene. 2000 kg.
hour. Regeneration of the catalyst is necessary after 300 of nitrobenzene and 1120 cubic meters (S.T.P) of fresh
hours, for example with a catalyst consisting of copper hydrogen are supplied per hour with the aid of a pump
applied to silicic acid at a throughput of about 150 kilo 30 to the two-component nozzles in uniform distribution. A
grams of nitrobenzene per kilogram of catalyst because pressure of 5 atomspheres gage is maintained in the re
after this period of Small amount of nitrobenzene, about action chamber. The reaction gas leaves the vessel
0.1%, appears in the reaction product. When using other through the said cyclone and passes through a heat ex
catalysts, other catalyst lives are achieved. In all cases, changer into a separating vessel in which the aniline
however, the life obtainable by the process according to formed and the water are separated from the gas. The
the present invention is longer than when the initial mate latter is returned through a preheater to the process with
rials are introduced into the fluidized layer exclusively the aid of a circulating pump. 2400 cubic meters (S.T.P.)
from below or from below and laterally at only one point. per hour of circulating gas are supplied to the vessel below
Alkylated nitrobenzenes, such as nitrotoluene or nitroxyl the distributor grate.
ene, may also be used as initial materials. 40 Cooling tubes parallel to the vessel wall and at a dis
The invention is illustrated by but not limited to the tance of 30 cm. therefrom are arranged in the lower two
following examples. thirds of the quiescent catalyst layer. These make it
Example I possible to maintain a temperature of 280° to 290° C.
A vertical reaction vessel consisting of a cylindrical tube
1700 kg. of water per hour are recovered in the cooling
System in the form of steam at 12 atmospheres gage.
5 meters in height and having a diameter of 200 mm. is 45
1510 kg. per hour of aniline are obtained in the separator
charged with 75 liters of a catalyst which consists of active (i.e., 99.5% of the theoretical yield) and 580 kg. of
alumina with 10% of copper and has a mean grain size Water. By simple distillation it is obtained with a purity
of 0.3 mm. The catalyst is supported by a grate through of nearly 100% and a content of nitrobenzene of less
which 12 cubic meters (S.T.P.) per hour of hydrogen are than 0.01%. It is only after an operational period of
introduced, the grains of catalyst thus being fluidized. more than 300 hours that the nitrobenzene content of the
Three nozzles are uniformly distributed at distances of 1, aniline reaches 0.05%. The original activity of the cat
2 and 3 meters from the bottom of the vessel and through alyst can be restored by regeneration with air at 350° C.
each of these 25 kilograms of liquid nitrobenzene flow and Subsequent reduction in the way described above. The
together with 14 cubic meters (S.T.P.) of hydrogen. The total life of the catalyst is 4 months.
injection nozzles are arranged so that the nitrozenzene 55
with the aid of hydrogen passes into the vessel as a fine
Similar results are obtained by using 7% of nickel
mist. An evaporator tube fed with water is situated in
instead of copper and 10% of molybdenum instead of
the vessel and withdraws the reaction heat of about 900
chromium, barium and zinc.
Kcals per kg. of nitrogen, thus making possible the main Example 3
tenance of a reaction temperature of 275 C. When the 60
A globular catalyst consisting of active alumina to
activity of the catalyst subsides, the temperature is grad
ually raised to about 400 C. When the first traces of which 18% of copper have been applied is placed in a
nitrobenzene appear in the aniline, the catalyst is re cylindrical reaction vessel having a diameter of 2 meters.
generated with air and then reduced in a current of hy Eleven nozzles are arranged in the layer of catalyst in
drogen at 250° C. Regeneration can be carried out ten the following way. At a height of 0.5 meter above the
times. The vapors and gases leaving the reaction vessel grate four nozzles are situated, after another 0.5 meter
are passed through a water cooler to a separating vessel. another four nozzles and after a further 0.5 meter three
Separation of gases from liquid takes place therein. An nozzles. Through these nozzles, 800 kg. of liquid nitro
aniline free from nitrobenzene is obtained in a purity of benzene per hour together with 1400 cubic meters
99.5%. The gas containing hydrogen is returned. 70 (S.T.P.) of a gas containing 90% of hydrogen are in
Instead of active alumina, magnesium oxide may be troduced in uniform dispersion. Below the grate, 13,000
used in a similar manner. cubic meters (S.T.P.) of circulating gas consisting of 50%
Example 2 of H2 hydrogen and 50% of N2 nitrogen are introduced
together with 800 liters of vaporized nitrobenzene. The
To carry out the reaction, a cylindrical reaction vessel 75 reaction pressure is 10 atomspheres gage. A cooling sys
3,186,818
5 6
tem is provided in the fluidized bed and this maintains benzene together with at least part of the hydrogen neces
the temperature at 300° C. The vapors escape at the up sary for the hydrogenation at a plurality of points, at least
per end of the reaction vessel, pass through a heat ex some of which are at different heights in the fluidized
changer in which they give up their heat to the circulating layer and are separated from each other by a vertical
hydrogen-containing gas and pass into a separating ves distance of at least 0.3 meter.
sel. The bulk of the hydrogen-containing gas is returned, 3. In a process for the production of aniline by hydro
a certain percentage being withdrawn in order to main genation of nitrobenzene with a substance selected from
tain an H2 concentration of 50% in the circulating gas. the class consisting of hydrogen and a hydrogen-contain
Aniline is obtained in a yield of 99.5%. The life of ing gas in the presence of a catalyst in a fluidized layer,
the catalyst before it needs regeneration is 400 to 600 O the improvement of introducing at least some of the nitro
hours and the total life is 7 months. benzene at a plurality of points, at least some of which
What we claim is: are at different heights in the fluidized layer and are
1. In a process for the production of aniline by hydro separated from each other by a vertical distance of at
genation of nitrobenzene with a substance selected from least 0.3 meter, and introducing the hydrogen necessary
the class consisting of hydrogen and a hydrogen-contain 5 for the hydrogenation at the bottom of the vessel.
ing gas in the presence of a catalyst in a fluidized layer, 4. A process as claimed in claim 1 which is carried out
the improvement of introducing the initial material to be under a pressure of from 7 to 25 atmospheres.
hydrogenated in at least partly liquid phase into the References Cited in the file of this patent
fluidized layer at a plurality of points at least some of
which are at different heights and are separated from each 20 UNITED STATES PATENTS
other by a vertical distance of at least 0.3 meter. 2,432,087 Brown ----------------- Dec. 9, 1947
2. In a process for the production of aniline by hydro OTHER REFERENCES
genation of nitrobenzene with a substance selected from
the class consisting of hydrogen and a hydrogen-contain "Fluid-Bed Catalyst-Solid Base for Boosting Aniline
ing gas in the presence of a catalyst in a fluidized layer, 25 Output Efficiency,” Chemical Week, vol. 85, Sept. 1959,
the improvement of introducing at least part of the nitro pages 68-70 and 72; 4 pages.
UNITED STATES PATENT OFFICE
CERTIFICATE OF CORRECTION
Patent No. 3 l36, 818 June 9, 1964
Heinrich Sperber et als
It is hereby certified that error appears in the above numbered pat
ent requiring
corrected correction and that the said Letters Patent should read as
below.
Column 2, line 56, for "100-250" read -- 100-250% --.
Signed and sealed this 10th day of November 1964.
(SEAL)
Attest:
ERNEST W. SWIDER EDWARD J. BRENNER
At testing Officer Commissioner of Patents
Вам также может понравиться
- United States Patent Office: Patented Feb. 6, 1951Документ3 страницыUnited States Patent Office: Patented Feb. 6, 1951karmilaОценок пока нет
- United States Patent Office: Patented Apr. 7, 1953Документ3 страницыUnited States Patent Office: Patented Apr. 7, 1953Syahrul SandreaОценок пока нет
- United States Patent Office: Patented Dec. 18, 1945Документ3 страницыUnited States Patent Office: Patented Dec. 18, 1945Jarukit Jr JunjiewchaiОценок пока нет
- Us 2498806Документ7 страницUs 2498806regina pramuditaОценок пока нет
- Proceeding of The Fertilizer Industry Round TableДокумент32 страницыProceeding of The Fertilizer Industry Round TableKhánh ĐỗОценок пока нет
- Jan. 22, 1963 L.. Domash Etal 3,074,380: Improvement 1N Hydrodesulfurization With Filed Dec. 30, 1958Документ5 страницJan. 22, 1963 L.. Domash Etal 3,074,380: Improvement 1N Hydrodesulfurization With Filed Dec. 30, 1958deni.sttnОценок пока нет
- Copie de US2899444-1Документ4 страницыCopie de US2899444-1KHALED KHALEDОценок пока нет
- Nov. 11, 1958 E. K. Jones Et Al: Manufacture of Isopropyl Benzene by Alkylation Filed JanДокумент8 страницNov. 11, 1958 E. K. Jones Et Al: Manufacture of Isopropyl Benzene by Alkylation Filed JanRizal EffendiОценок пока нет
- S SSS L, SS S SS SN S SS S: March 19, 1968 N. L. Carr Etal 3,374,280Документ4 страницыS SSS L, SS S SS SN S SS S: March 19, 1968 N. L. Carr Etal 3,374,280toastcfhОценок пока нет
- Sept. 25, 1951 L. A. Stenge 2,568,901: Eles. 44tagedДокумент4 страницыSept. 25, 1951 L. A. Stenge 2,568,901: Eles. 44tagedwakanda foreverОценок пока нет
- Activated ClayДокумент7 страницActivated ClayvietpineОценок пока нет
- Raney Ni PurificationДокумент5 страницRaney Ni Purificationanjireddy1612Оценок пока нет
- Patent Benzil KloridaДокумент3 страницыPatent Benzil KloridaShochib Al FatihОценок пока нет
- Chemical and Mechanical DesignДокумент460 страницChemical and Mechanical DesignNuriman K-monОценок пока нет
- Catalysts: Platinum in OxidationДокумент5 страницCatalysts: Platinum in Oxidationdshree5811Оценок пока нет
- US3012862 Si Halide With Hydrogen PatentДокумент7 страницUS3012862 Si Halide With Hydrogen PatentLiya Elizabeth JacobОценок пока нет
- United States Patent Office: Patented June 23, 1970Документ2 страницыUnited States Patent Office: Patented June 23, 1970MãdãlinaStanciuОценок пока нет
- ' United States - Patent Office: Patented Nov. 15, 1949Документ7 страниц' United States - Patent Office: Patented Nov. 15, 1949nazanin timasiОценок пока нет
- Chemical Reactors: Batch Reactors Are Used For Most of The Reactions Carried Out in A Laboratory. The Reactants AreДокумент4 страницыChemical Reactors: Batch Reactors Are Used For Most of The Reactions Carried Out in A Laboratory. The Reactants AreBranco RojasОценок пока нет
- Butane Oxidation To Maleic Anhydride Kin PDFДокумент13 страницButane Oxidation To Maleic Anhydride Kin PDFPrasad ShahОценок пока нет
- Fractionation of Petroleum: Electric Desalting & DistillationДокумент25 страницFractionation of Petroleum: Electric Desalting & DistillationAnand kesanakurtiОценок пока нет
- United States Patent Office: Patented Mar. 21, 1950Документ2 страницыUnited States Patent Office: Patented Mar. 21, 1950alexОценок пока нет
- United States Patent: 1,251,202 12/1917 Ellis.............................. 260/667Документ6 страницUnited States Patent: 1,251,202 12/1917 Ellis.............................. 260/667Septi WidyaОценок пока нет
- Nitro Benzene Sai PDFДокумент65 страницNitro Benzene Sai PDFDeepak Pandey100% (1)
- United States Patent (19) : Hu Et AlДокумент4 страницыUnited States Patent (19) : Hu Et AlSepti WidyaОценок пока нет
- Process of Production CS2 PDFДокумент9 страницProcess of Production CS2 PDFDinii Lathiifah PertiwiОценок пока нет
- Process Control LДокумент23 страницыProcess Control Ltariq fareedОценок пока нет
- OF TO: Oxidation Acetaldehyde Acetic Acid In1 A Sparger ReactorДокумент8 страницOF TO: Oxidation Acetaldehyde Acetic Acid In1 A Sparger Reactorarpit gargОценок пока нет
- Fluidized Bed Reactor For The Hydrogenation of Nitrobenzene To AnilineДокумент21 страницаFluidized Bed Reactor For The Hydrogenation of Nitrobenzene To AnilineJeff Gomez PerezОценок пока нет
- Us 3642838 PatentДокумент3 страницыUs 3642838 PatentElsie XiaoОценок пока нет
- Abstracts of The Papers Presented at The Thirteenth National Vacuum SymposiumДокумент1 страницаAbstracts of The Papers Presented at The Thirteenth National Vacuum SymposiumgfpeezyОценок пока нет
- Thorium-Fueled Underground Power Plant Based On Molten Salt TechnologyДокумент7 страницThorium-Fueled Underground Power Plant Based On Molten Salt Technologywsteffen33Оценок пока нет
- Polyphosphonitrilic Chloride: 1. ProcedureДокумент3 страницыPolyphosphonitrilic Chloride: 1. ProcedureAfrah MОценок пока нет
- Process and Apparatus For The Pyrolysis of Organic CompoundsДокумент4 страницыProcess and Apparatus For The Pyrolysis of Organic CompoundsAОценок пока нет
- Produksi Phthalic AnhydrideДокумент5 страницProduksi Phthalic Anhydridehalim syarifОценок пока нет
- Equipment For Production of An Alkylation Plant For Igh-Octane GasolineДокумент5 страницEquipment For Production of An Alkylation Plant For Igh-Octane GasolinejoefrizalОценок пока нет
- United States Patent (15) 3,697,598: Leif Urban Folke Thorsen, Örn Primary Examiner-Retiri RaymondДокумент7 страницUnited States Patent (15) 3,697,598: Leif Urban Folke Thorsen, Örn Primary Examiner-Retiri RaymondSalsabila CacaОценок пока нет
- Optimization of The Naphtha Hydro Treating Unit (NHT) in Order To Increase Feed in The RefineryДокумент12 страницOptimization of The Naphtha Hydro Treating Unit (NHT) in Order To Increase Feed in The RefineryvivekchateОценок пока нет
- Heat Treating Catalyst Improves Arylnitroalkene ReductionДокумент5 страницHeat Treating Catalyst Improves Arylnitroalkene ReductionFlorian FischerОценок пока нет
- Polyphosphonitrilic Chloride: 1. ProcedureДокумент3 страницыPolyphosphonitrilic Chloride: 1. ProcedureAfrah MОценок пока нет
- MANUFACTURING METHODS OF SULFURIC ACIDДокумент11 страницMANUFACTURING METHODS OF SULFURIC ACIDZamir Khan100% (3)
- Qdoc - Tips Chemical Engg ReviewerДокумент53 страницыQdoc - Tips Chemical Engg ReviewerMa Theresa CabiazaОценок пока нет
- Manufacturing Proceses for Styrene ProductionДокумент9 страницManufacturing Proceses for Styrene ProductionMohd Zulazreen50% (2)
- Recrystallization Process Upgrades Rock and Solar SaltsДокумент17 страницRecrystallization Process Upgrades Rock and Solar SaltsVirgil CenariuОценок пока нет
- Thorium-Fueled Underground Power PlantДокумент7 страницThorium-Fueled Underground Power Plantalfonico100% (1)
- US2221622 Purification of ManganeseДокумент2 страницыUS2221622 Purification of ManganeserichardОценок пока нет
- US2770525Документ2 страницыUS2770525Wojciech RedutkoОценок пока нет
- US1938609Документ3 страницыUS1938609分析室信箱Оценок пока нет
- Producing Granular Ammonium Nitrate Using Heat of NeutralizationДокумент9 страницProducing Granular Ammonium Nitrate Using Heat of NeutralizationAhmed ShaalanОценок пока нет
- Leaching Section 3Документ16 страницLeaching Section 3EDSON CHENJERAIОценок пока нет
- v111n11p759 Fluid Roasting ZincДокумент7 страницv111n11p759 Fluid Roasting ZincergfaradОценок пока нет
- Chemical Engg ReviewerДокумент53 страницыChemical Engg ReviewerJasonTenebroso100% (2)
- Different Zones in The Reactor-Chemistry of GasificationДокумент6 страницDifferent Zones in The Reactor-Chemistry of GasificationN.R. RishiОценок пока нет
- Study of Propane Dehydrogenation To Propylene in An Integrated Fluidized Bed Reactor Using Pt-Sn/Al-SAPO-34 Novel CatalystДокумент6 страницStudy of Propane Dehydrogenation To Propylene in An Integrated Fluidized Bed Reactor Using Pt-Sn/Al-SAPO-34 Novel CatalystDevika JayapalОценок пока нет
- United States Patent Office: As The Central Atom On A CarrierДокумент4 страницыUnited States Patent Office: As The Central Atom On A CarrierRasoulОценок пока нет
- US3303001Документ3 страницыUS3303001Lokesh RavichandranОценок пока нет
- United States Patent 1191: Tu (45) Sep. 7, 1982Документ4 страницыUnited States Patent 1191: Tu (45) Sep. 7, 1982AdyОценок пока нет
- Encyclopaedia Britannica, 11th Edition, Volume 8, Slice 3 "Destructors" to "Diameter"От EverandEncyclopaedia Britannica, 11th Edition, Volume 8, Slice 3 "Destructors" to "Diameter"Оценок пока нет
- Direct Conversion of Syngas To Chemicals Using Heterogeneous CatalystsДокумент6 страницDirect Conversion of Syngas To Chemicals Using Heterogeneous CatalystsJoel Flores CastilloОценок пока нет
- Formaldehyde by Ag OxideДокумент12 страницFormaldehyde by Ag OxideVinh Do ThanhОценок пока нет
- Processes: Performance Comparison of Industrially Produced Formaldehyde Using Two DiДокумент12 страницProcesses: Performance Comparison of Industrially Produced Formaldehyde Using Two DiMohammed FaiqОценок пока нет
- Nanoscience and Nanotechnology in IndiaДокумент8 страницNanoscience and Nanotechnology in IndiaJoel Flores CastilloОценок пока нет
- US3136818-Produccion de AnilinaДокумент4 страницыUS3136818-Produccion de AnilinaJoel Flores CastilloОценок пока нет
- Reuss, Günther - Ullmann's Encyclopedia of Industrial ChemistryДокумент34 страницыReuss, Günther - Ullmann's Encyclopedia of Industrial ChemistryVeny Nofitasary100% (1)
- Rules of Thumb Ludwigs Applied Process PDFДокумент9 страницRules of Thumb Ludwigs Applied Process PDFAngelena Rani FrancisОценок пока нет
- Ref Ohsas 18001Документ7 страницRef Ohsas 18001norliyatiОценок пока нет
- Turton AppBДокумент114 страницTurton AppBamms9988Оценок пока нет
- AstmДокумент3 страницыAstmMuhammad NaumanОценок пока нет
- A Review of The Present and Future Utilisation of FRP Composites in The Civil Infrastructure With Reference To Their Important In-Service PropertiesДокумент27 страницA Review of The Present and Future Utilisation of FRP Composites in The Civil Infrastructure With Reference To Their Important In-Service PropertiesalbertofgvОценок пока нет
- URA Elect SR 2005 03Документ55 страницURA Elect SR 2005 03Zhu Qi WangОценок пока нет
- SHOP MANUAL Sen00203-18 D475Документ1 617 страницSHOP MANUAL Sen00203-18 D475Wilfredo Escobar Gutierrez100% (3)
- Probeta JiskootДокумент2 страницыProbeta JiskootFrank Mathews GОценок пока нет
- Pec RulesДокумент2 страницыPec RulesMhayson LpaguipoОценок пока нет
- Ford DTCДокумент35 страницFord DTCAmina K. KhalilОценок пока нет
- Introductory Overview: Diagram of A Typical Coal-Fired Thermal Power StationДокумент9 страницIntroductory Overview: Diagram of A Typical Coal-Fired Thermal Power StationVV CommunicationsОценок пока нет
- Cast Iron: Cast Irons Are A Class of Ferrous Alloys WithДокумент12 страницCast Iron: Cast Irons Are A Class of Ferrous Alloys WithChandima K PriyamalОценок пока нет
- Cam Lact Data SheetДокумент4 страницыCam Lact Data SheetYana ParravanoОценок пока нет
- Panasonic TC-P42X5 Service ManualДокумент74 страницыPanasonic TC-P42X5 Service ManualManager iDClaimОценок пока нет
- Diesel Engineering Handbook Cooling SystemsДокумент14 страницDiesel Engineering Handbook Cooling Systemsmartin.ruben100% (1)
- PDK 205481 KW26-S5-FSE-4Q enДокумент83 страницыPDK 205481 KW26-S5-FSE-4Q enRoberto SacotoОценок пока нет
- Certification AbbreviationsДокумент3 страницыCertification AbbreviationsAmine DabbabiОценок пока нет
- Soxhelet Extraction of Crude DrugsДокумент38 страницSoxhelet Extraction of Crude DrugsManjula Raghu0% (1)
- Xpelair GX6 (90800AW) 6 Inch Axial Extract Fans Spec1Документ2 страницыXpelair GX6 (90800AW) 6 Inch Axial Extract Fans Spec1Augustine Dharmaraj100% (1)
- Cutting ToolsДокумент143 страницыCutting ToolsTone RatanalertОценок пока нет
- Astm A681 PDFДокумент14 страницAstm A681 PDFraulОценок пока нет
- TC-Lube Technical-Brochure DSi May2012 PDFДокумент8 страницTC-Lube Technical-Brochure DSi May2012 PDFbourbiaОценок пока нет
- Materials Selection Assignment. LiveДокумент10 страницMaterials Selection Assignment. Liverichward5Оценок пока нет
- Mole ConceptДокумент3 страницыMole Conceptzafarchem_iqbalОценок пока нет
- KURADДокумент1 страницаKURADbeto pagoadaОценок пока нет
- Hazardous Waste FlowchartДокумент21 страницаHazardous Waste FlowchartRizki Mufidayanti100% (1)
- Silicon NPN Triple Diffusion Mesa Type: Power TransistorsДокумент3 страницыSilicon NPN Triple Diffusion Mesa Type: Power TransistorsCatarroCatarroОценок пока нет
- Fineotex Chemical LimitedДокумент46 страницFineotex Chemical LimitedINDUDEVIОценок пока нет
- 55 Emerson Catalog PDFДокумент82 страницы55 Emerson Catalog PDFBRLSwamyОценок пока нет
- مهم٢Документ127 страницمهم٢سجى وليدОценок пока нет
- Vivax Acp-12ch35gei Service ManualДокумент67 страницVivax Acp-12ch35gei Service Manualdrm_gОценок пока нет
- Bearing Design and Metallurgy GuideДокумент46 страницBearing Design and Metallurgy GuideKumarОценок пока нет
- Roman Blacksmithing TechniquesДокумент11 страницRoman Blacksmithing TechniquesaoransayОценок пока нет