Академический Документы
Профессиональный Документы
Культура Документы
Steps in tech transfer process
Загружено:
William DC RiveraИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Steps in tech transfer process
Загружено:
William DC RiveraАвторское право:
Доступные форматы
M3 - Lesson 2 Steps in technology transfer
Steps in technology transfer
Technology Transfer is not a single way process. Whether a tablet, a Transdermal patch, a topical
ointment, or an injectable, the transformation of a pharmaceutical prototype into a successful product
requires the cooperation of many individuals.
The classic view of a flow from basic to applied technology is a great oversimplification-sometimes, e.g.
problems or insights arising at the production level give rise to new ideas that contribute to fundamental
basic advance. At least in some sectors, close links between the basic researchers and manufacturing
experts, and even marketing personnel contribute to competitiveness and advancement.
During development of a formulation, it is important to understand procedure of operations used,
critical and non-critical parameters of each operation, production environment, equipment and
excipient availability, which should be taken into account during the early phases of development of
formulation, so that successful scale up can be carried out. Appropriate care during technology transfer
is important to enhance drug quality as developed by R & D in final formulation as well as to assure
quality for predetermined period of time. The various steps involved in technology transfer are given
below:
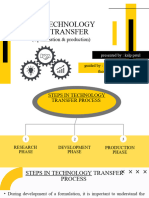
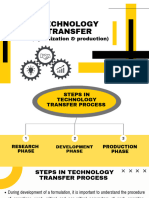
A. Development of technology by R & D [Research Phase]
B. Technology transfer from R&D to production [Development Phase]
C. Optimization and Production [Production Phase]
D. Technology Transfer Documentation
Вам также может понравиться
- Technology Transfer in Pharmaceutical Industry Objective, Issues and Policy ApproachesДокумент6 страницTechnology Transfer in Pharmaceutical Industry Objective, Issues and Policy ApproachesLuis Arturo Pineda Vergara0% (1)
- TT ForwardДокумент28 страницTT ForwardKeshavTiwariОценок пока нет
- Technology Transfer From R&D To ProductionДокумент16 страницTechnology Transfer From R&D To ProductionRutuja Chougale100% (1)
- Technology TransferДокумент58 страницTechnology TransferVaishali YardiОценок пока нет
- Technology Transfer in IndiaДокумент9 страницTechnology Transfer in IndiaMehak Bhargava100% (1)
- Tech Transfer 1Документ6 страницTech Transfer 1Rajesh KesarlaОценок пока нет
- Technology Transfer Embodies Both The Transfer of Documentation and The Demonstrated Ability of The Receiving UnitДокумент18 страницTechnology Transfer Embodies Both The Transfer of Documentation and The Demonstrated Ability of The Receiving UnitkoolmanissshОценок пока нет
- Technology Transfer in Pharmaceutical IndustryДокумент5 страницTechnology Transfer in Pharmaceutical IndustryGursharanjit Singh ShinhОценок пока нет
- Tech TransferДокумент51 страницаTech Transferkunalprabhu148Оценок пока нет
- Innovation ManagementДокумент37 страницInnovation ManagementChandni GautamОценок пока нет
- Innovation ManagementДокумент33 страницыInnovation ManagementNavi SinghОценок пока нет
- Technology TransferДокумент68 страницTechnology TransferPhúc CaoОценок пока нет
- Transfer Rifaximin Tablet TechДокумент49 страницTransfer Rifaximin Tablet TechSujit DasОценок пока нет
- Technology Transfer by KunalДокумент18 страницTechnology Transfer by KunalRohit ShirsathОценок пока нет
- Technology Transfer by KunalДокумент18 страницTechnology Transfer by KunalRohit ShirsathОценок пока нет
- BP702T Ip IiДокумент29 страницBP702T Ip IiTurbo ChargedОценок пока нет
- BP702T Ip IiДокумент29 страницBP702T Ip IiMukund JhaОценок пока нет
- TRANSFER OF TECHNOLOGY ManuДокумент4 страницыTRANSFER OF TECHNOLOGY Manuheyyo ggОценок пока нет
- Unit 2 Industrial Pharmacy 2 7th SemesterДокумент29 страницUnit 2 Industrial Pharmacy 2 7th SemesterDurgha SureshОценок пока нет
- 619Документ3 страницы619elektron2010Оценок пока нет
- Exploitation TM Chapter 2Документ7 страницExploitation TM Chapter 2kainat khalidОценок пока нет
- 3812-Article Text-10914-1-10-20170219Документ7 страниц3812-Article Text-10914-1-10-20170219harini_kОценок пока нет
- Chapter 2Документ15 страницChapter 2mehnoor kaurОценок пока нет
- Articulo Tecnologia de TransferenciaДокумент10 страницArticulo Tecnologia de Transferencia20172504Оценок пока нет
- Technology Transfer - 20230927 - 114610 - 0000Документ10 страницTechnology Transfer - 20230927 - 114610 - 0000KALP PATELОценок пока нет
- Qualitative and Quantitative Models of Technology TransferДокумент46 страницQualitative and Quantitative Models of Technology TransferABHIJEET KOKATE100% (1)
- Comparative Characteristics of TQM and ReengineeringДокумент5 страницComparative Characteristics of TQM and ReengineeringMilan KolarevicОценок пока нет
- MS 94Документ9 страницMS 94Rajni KumariОценок пока нет
- Technology Transfer in Pharmaceutical IndustryДокумент7 страницTechnology Transfer in Pharmaceutical Industryvedant chaudhariОценок пока нет
- IJPSR (2013), Vol. 4, Issue 5 (Review Article) : ISSN: 0975-8232Документ17 страницIJPSR (2013), Vol. 4, Issue 5 (Review Article) : ISSN: 0975-8232Nitin SheokandОценок пока нет
- Technology Transfer 1Документ5 страницTechnology Transfer 1Android modded gamesОценок пока нет
- Technology Management BBA AssignmentДокумент12 страницTechnology Management BBA AssignmentNageshwar SinghОценок пока нет
- Comparative Characteristics of TQM and ReengineeringДокумент5 страницComparative Characteristics of TQM and ReengineeringAndrei SerbanОценок пока нет
- Be-Unit 369Документ78 страницBe-Unit 369Anisha BhatiaОценок пока нет
- How Tech Innovation Is Transforming Business StrategiesДокумент6 страницHow Tech Innovation Is Transforming Business StrategiesJahnaviОценок пока нет
- Review of The Strategies For Technology Transfer in Manufacturing SectorДокумент6 страницReview of The Strategies For Technology Transfer in Manufacturing SectoraijbmОценок пока нет
- Ashleen Akengo VitoloДокумент9 страницAshleen Akengo VitoloMayi PennylОценок пока нет
- MI0031 Technology Management FALL 2010 Set 2Документ4 страницыMI0031 Technology Management FALL 2010 Set 2Yogesh VermaОценок пока нет
- Notes On Management of Technology & Innovation (Different Sources) - SteemitДокумент26 страницNotes On Management of Technology & Innovation (Different Sources) - SteemitDexter LaboratoryОценок пока нет
- Flexpro Whitepaper Guide To A Successful Drug Development Technology Transfer 1Документ12 страницFlexpro Whitepaper Guide To A Successful Drug Development Technology Transfer 1zakarya wadiОценок пока нет
- Gnipst 4 30 5694 2.2Документ2 страницыGnipst 4 30 5694 2.2Antar GayenОценок пока нет
- Gnipst 4 30 5694 2.1Документ5 страницGnipst 4 30 5694 2.1Antar GayenОценок пока нет
- BBA601 SLM Unit 07 PDFДокумент22 страницыBBA601 SLM Unit 07 PDFSarah SajjadОценок пока нет
- From Single To A Multiproduct Facility, Key ConsiderationsДокумент8 страницFrom Single To A Multiproduct Facility, Key ConsiderationsCathal DoranОценок пока нет
- Innovation Management How Ensure Business Competitiveness SustainabilityДокумент25 страницInnovation Management How Ensure Business Competitiveness SustainabilityRached LazregОценок пока нет
- Gr4 Bpo Written ReportДокумент16 страницGr4 Bpo Written ReportEricka Joy Torres100% (1)
- Forum Discussion KanggadeviДокумент5 страницForum Discussion KanggadeviKanggadevi MurthiОценок пока нет
- Technological Environment: - Debashree MukherjeeДокумент9 страницTechnological Environment: - Debashree Mukherjeeshahid_ashrafОценок пока нет
- Technology Transfer - 20230918 - 201200 - 0000Документ9 страницTechnology Transfer - 20230918 - 201200 - 0000KALP PATELОценок пока нет
- Management of Innovation & Technology: Introduction & OverviewДокумент33 страницыManagement of Innovation & Technology: Introduction & OverviewPrincess Marshalee FosterОценок пока нет
- Unit 1 ItmДокумент9 страницUnit 1 ItmNITESH SONIОценок пока нет
- Technological EnvironmentДокумент10 страницTechnological EnvironmentNandini MukherjeeОценок пока нет
- Framework for Understanding TM Activities and ToolsДокумент28 страницFramework for Understanding TM Activities and ToolssanaОценок пока нет
- Lecture 6, 7Документ27 страницLecture 6, 7deeptitripathi09Оценок пока нет
- Unit IiiДокумент11 страницUnit IiiMarienelle De La CruzОценок пока нет
- Pilot Plant Scale Up TechniquesДокумент32 страницыPilot Plant Scale Up TechniquesDo Something GoodОценок пока нет
- Technology Transfer: What ? How ?: Hopefully You Should Be Able To Answer These Questions at The End of This SeminarДокумент38 страницTechnology Transfer: What ? How ?: Hopefully You Should Be Able To Answer These Questions at The End of This Seminarm.asuncionОценок пока нет
- Optimize Performance. Engineer Success.: Landmark Services White PaperДокумент12 страницOptimize Performance. Engineer Success.: Landmark Services White PaperSagar KalraОценок пока нет
- M3-Lesson 3 - Media & Social ChangeДокумент2 страницыM3-Lesson 3 - Media & Social ChangeWilliam DC RiveraОценок пока нет
- M5-Lesson 2 - Media Industry and The Social WorldДокумент2 страницыM5-Lesson 2 - Media Industry and The Social WorldWilliam DC RiveraОценок пока нет
- M3-Lesson 1-Part 2Документ2 страницыM3-Lesson 1-Part 2William DC RiveraОценок пока нет
- M2-Lesson 3 Part 2Документ2 страницыM2-Lesson 3 Part 2William DC RiveraОценок пока нет
- M3-Lesson 2 - Media & Social ChangeДокумент2 страницыM3-Lesson 2 - Media & Social ChangeWilliam DC RiveraОценок пока нет
- M3-Lesson 1-Media & Social ChangeДокумент2 страницыM3-Lesson 1-Media & Social ChangeWilliam DC RiveraОценок пока нет
- M2-Lesson 2-Media & Other Social InstitutionsДокумент2 страницыM2-Lesson 2-Media & Other Social InstitutionsWilliam DC RiveraОценок пока нет
- M2-Introduction - Media & Other Social InstitutionsДокумент2 страницыM2-Introduction - Media & Other Social InstitutionsWilliam DC RiveraОценок пока нет
- M4 Post TaskДокумент1 страницаM4 Post TaskWilliam DC RiveraОценок пока нет
- M3-Lesson 1-Part 3Документ2 страницыM3-Lesson 1-Part 3William DC RiveraОценок пока нет
- M1 Lesson 3 - Media & The Social WorldДокумент1 страницаM1 Lesson 3 - Media & The Social WorldWilliam DC RiveraОценок пока нет
- M2-Lesson 3 - Media & Other Social InstitutionsДокумент2 страницыM2-Lesson 3 - Media & Other Social InstitutionsWilliam DC RiveraОценок пока нет
- M5-Lesson 3 - Media Industry and The Social WorldДокумент1 страницаM5-Lesson 3 - Media Industry and The Social WorldWilliam DC RiveraОценок пока нет
- M5-Lesson 1 - Media Industry and The Social WorldДокумент1 страницаM5-Lesson 1 - Media Industry and The Social WorldWilliam DC RiveraОценок пока нет
- M4-Lesson 1-Media Representations of The Social WorldДокумент1 страницаM4-Lesson 1-Media Representations of The Social WorldWilliam DC RiveraОценок пока нет
- M2-Lesson 1-Media & Other Social InstitutionsДокумент2 страницыM2-Lesson 1-Media & Other Social InstitutionsWilliam DC RiveraОценок пока нет
- Do You Agree With The Argument of Symbolic Interactionism? Why? Briefly Explain Your OpinionДокумент1 страницаDo You Agree With The Argument of Symbolic Interactionism? Why? Briefly Explain Your OpinionWilliam DC RiveraОценок пока нет
- M5 Post TaskДокумент1 страницаM5 Post TaskWilliam DC RiveraОценок пока нет
- M4 While TaskДокумент1 страницаM4 While TaskWilliam DC RiveraОценок пока нет
- At The End of This Module, You Should Be Able To:: M5 ObjectivesДокумент1 страницаAt The End of This Module, You Should Be Able To:: M5 ObjectivesWilliam DC RiveraОценок пока нет
- M2 While Task (Let's Engage)Документ1 страницаM2 While Task (Let's Engage)William DC RiveraОценок пока нет
- M4-Lesson 2-Media Representations of The Social WorldДокумент2 страницыM4-Lesson 2-Media Representations of The Social WorldWilliam DC RiveraОценок пока нет
- M5 While TaskДокумент1 страницаM5 While TaskWilliam DC RiveraОценок пока нет
- M3 Pre TaskДокумент1 страницаM3 Pre TaskWilliam DC RiveraОценок пока нет
- M5 Pre-Task 2323 Unread Replies.2828 RepliesДокумент1 страницаM5 Pre-Task 2323 Unread Replies.2828 RepliesWilliam DC RiveraОценок пока нет
- Previous Next: in Your Own Words, Critically Evaluate The Ideas of Marx, Mead, and Luhmann About Social ChangeДокумент1 страницаPrevious Next: in Your Own Words, Critically Evaluate The Ideas of Marx, Mead, and Luhmann About Social ChangeWilliam DC RiveraОценок пока нет
- M2 Pre Task (Reflection)Документ1 страницаM2 Pre Task (Reflection)William DC RiveraОценок пока нет
- "The Philosophers Have Interpreted The World, in Various Ways The Point Is To Change It." (Theses OnДокумент1 страница"The Philosophers Have Interpreted The World, in Various Ways The Point Is To Change It." (Theses OnWilliam DC RiveraОценок пока нет
- M3 ObjectiveДокумент1 страницаM3 ObjectiveWilliam DC RiveraОценок пока нет
- Sentences Answer Here.: Identity One TV Show That Represent Filipino Family. How Does It Depict The Family? Share Your 5Документ1 страницаSentences Answer Here.: Identity One TV Show That Represent Filipino Family. How Does It Depict The Family? Share Your 5William DC RiveraОценок пока нет