Академический Документы
Профессиональный Документы
Культура Документы
Chapter 13 - Stereo Isomerism
Загружено:
Bhanu Pratap ChauhanИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Chapter 13 - Stereo Isomerism
Загружено:
Bhanu Pratap ChauhanАвторское право:
Доступные форматы
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 1
Chapter 13 Stereoisomerism
Topic 20 from the IB HL Chemistry Curriculum
Assessment Statement Describe stereoisomers as compounds with the same structural formula but with different arrangements of atoms in space. Describe and explain geometrical isomerism in non-cyclic alkenes. Describe and explain geometrical isomerism in C3 and C4 cycloalkanes. Explain the difference in the physical and chemical properties of geometrical isomers. Obj 2 Teachers Notes 20.6.1
20.6.2
Include the prefixes cis- and trans- and the term restricted rotation.
20.6.3
Include the dichloro derivatives of cyclopropane and cyclobutane.
20.6.4
Include cis- and trans-1,2-dichloroethene as examples with different boiling points, and cis- and trans-but-2-ene-1,4-dioic acid as examples that react differently when heated. Include examples such as butan-2-ol and 2-bromobutane. The term asymmetric can be used to describe a carbon atom joined to four different atoms or groups, and also as a description of the molecule itself. Include the meanings of the terms enantiomer and racemic mixture. TOK: The existence of optical isomers provided indirect evidence of a tetrahedrally bonded carbon atom. This is an example of the power of reasoning in allowing use access to the molecular scale. Do we know or believe those carbon atoms are tetrahedrally coordinated? The use of conventions in representing three-dimensional molecules in two dimensions could also be discussed. Include the meaning of the term plane-polarized light.
20.6.5
Describe and explain optical isomerism in simple organic molecules.
20.6.6
Outline the use of a polarimeter in distinguishing between optical isomers. Compare the physical and chemical properties of enantiomers.
20.6.7
Stereoisomerism The structural isomers discussed in Chapter 03 differ from each other in the order of attachment of the atoms. Consequently, these are molecules which are fundamentally different from each other, having different amounts of branching in their chains or different positions of functional groups or even possessing entirely different functional groups. As we have seen, it is relatively easy to describe the differences between these isomers using structural formulas in two dimensions. However, another type of isomerism, known as stereoisomerism, is much harder to describe on paper. This is because these molecules have atoms attached together in the same order, but differ from each other in their spatial (threedimensional) arrangement. You will find that your understanding of this topic will be greatly helped by looking at and building three-dimensional models of the different molecules. There are two types of stereoisomerism that we will discuss here, geometric (now more commonly known as cis- trans), and optical isomers.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 2
Geometric isomers (cis-trans isomers) When there is some constraint in a molecule that restricts the free rotation of bonded groups, they become fixed in space relative to each other. So, where there are two different groups attached to each of the two carbon atoms that have restricted rotation, this gives rise to two different three-dimensional arrangements of the atoms known as geometric isomers. The restriction on rotation can be caused by a double bond or a cyclic structure as shown below.
Cis- refers to the isomer that has the same groups on the same side of the double bond or ring, while trans- is the isomer that has the same groups on opposite sides, or across the point of restricted rotation. These prefixes are given in italics before the name of the compound. Some examples of cis-trans isomerism in these circumstances are discussed below.
Double bond We learned in Chapter 11 that the double bond consists of one sigma and one pi bond, and that the pi bond forms by the sideways overlap of two p orbitals. Free rotation around this is not possible as it would push the p orbitals out of position; and the pi bond would break. For example:
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 3
Molecular graphics of the geometric isomers of 1,2-dibromoethene. The atoms are shown as color-coded cylinders, with carbon in yellow, hydrogen in white and bromine in red. The cis- form is on the left and the trans- form on the right. The molecule cannot alternate between the two forms as there is restricted rotation about the carbon-carbon double bond.
Worked example: Draw and name the geometric isomers of butenedioic acid. Solution: As the carboxylic acid groups must be in the terminal positions and cannot be attached to a double bond, the condensed structural formula must be HOOC-(CH)2-COOH. To identify the geometric isomers, we need to draw this out in full.
Cis- and trans-isomers have different properties depending on the influence of the substituted group in the molecule. Physical properties Physical properties depend on: the polarity of the molecules the shape or symmetry of the molecules.
The polarity strongly influences the relative boiling point as it determines the strength of the intermolecular forces. For example, cis-1,2-dichloroethene has a net dipole moment and dipole-dipole attractions between its molecules in addition to the van der Waals' forces, whereas trans-1,2-dichloroethene which is non-polar has only van der Waals' forces. The boiling point of the cis-isomer is therefore higher.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 4
Melting point is generally more influenced by the symmetry of the molecules as this affects the packing in the solid state. The trans-isomers are able to pack more closely due to their greater symmetry, so the intermolecular forces are more effective than in the cis-isomer. The melting point of the trans-isomer is therefore higher.
A similar comparison is seen with butenedioic acid where the melting point of the trans-isomer is significantly higher than that of the cis-isomer. Here the cis-isomer is able to form intramolecular hydrogen bonds between the two -COOH groups due to their close proximity, whereas in the trans-isomer the - COOH groups sticking out on opposite sides of the molecule are free to form intermolecular hydrogen bonds. Thus more energy is needed to separate the trans isomer molecules, hence the higher melting point.
Chemical properties Chemical properties of the cis- and trans- isomers are usually very similar, but an interesting exception to this is seen with butenedioic acid. Here the two isomers have such different reactivities, as shown by example below in their responses to being heated, that they were originally given different names.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 5
The differences in the properties of the cis-and trans- isomers of butenedioic acid become very evident when examples of their roles in biology are compared. Fumaric acid (trans-) is an intermediate in the Krebs cycle, an essential part of the reactions of aerobic respiration for energy release in cells. By contrast, maleic acid (cis-) is an inhibitor of reactions that interconvert amino acids, for example, in the human liver. Their different biological activities are a consequence of their different shapes affecting their binding to enzymes, the biological catalysts that control all these reactions.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 6
Cyclic molecules Cycloalkanes contain a ring of carbon atoms in which the bond angles are strained from the tetrahedral angles in the parent alkane. For example, in cyclopropane the carbon atoms form a triangle with bond angles of 60, and in cyclobutane the atoms form a puckered square with approximate angles of 90. The ring prevents rotation around the carbon atoms, so when there are two different groups attached to two carbons in the ring, these molecules can exist as the cis- and trans- forms. For example:
As you can see from the example of 1,3-dichlorocyclobutane above, the substituted groups do not have to be on adjacent carbon atoms; it is their position relative to the plane of the ring that defines the geometric isomer.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 7
Optical isomers A carbon atom attached to four different atoms or groups is known as asymmetric or chiral. The four groups, arranged tetrahedrally around the carbon atom with bond angles of 109.5, can be arranged in two different three-dimensional configurations which are mirror images of each other. This is known as optical isomerism. The term refers to the ways in which the isomers interact with plane-polarized light, discussed below. They are said to be chiral molecules and have no plane of symmetry. In the following figure, an asymmetric, or chiral, carbon atom, shown in black, is bonded to four different atoms or groups shown here in different colors. This gives rise to two configurations which are mirror images of each other.
The word chiral is derived from the Greek word for hand. Lord Kelvin first introduced the term into science in 1904 with the now celebrated definition: I call any geometrical figure, or group of points, chiral, and say it has chirality if its image in a plane mirror, ideally realized, cannot be brought to coincide with itself. His definition can therefore be applied much more generally to structures outside chemistry, such as knots. If you look at your two hands, you will see that they also are mirror images. When you put them directly on top of each other, the fingers and thumbs do not line up - we say they are non-superimposable.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 8
The same is true for optical isomers, and the two non-superimposable forms are known as enantiomers. A mixture containing equal amounts of the two enantiomers is known as a racemic mixture or a racemate. As we will see, such a mixture is said to be optically inactive. A single chiral centre in a molecule gives rise to two stereoisomers. ln general, a molecule with n chiral centres has a maximum of 2n stereoisomers, although some may be too strained to exist. For example, cholesterol has eight chiral centres and so a possible 28 = 256 stereoisomers. Only one is produced in biological systems. We can find optical activity in many of the molecules we have already encountered in this chapter. The clue is to look for any carbon atom that is bonded to four different groups. It is often useful to mark that carbon with an asterisk.
When you are looking for a chiral carbon atom in a molecule, you must look at the whole group bonded to the carbon, not just the immediately bonded atom for example CH3 is a different group from C2H5.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 9
Worked example: Draw the enantiomers of 2-hydroxypropanoic acid (lactic acid). Mark the chiral carbon atom and show the plane of the mirror. Solution First draw out the full structure and identify the chiral carbon atom.
Properties of optical isomers Optical isomers, the enantiorners, have identical physical and chemical properties with two important exceptions: optical activity reactivity with other chiral molecules Optical activity As we know from their name, optical isomers show a difference in a specific interaction with light. A beam of ordinary light consists of electromagnetic waves that oscillate in an infinite number of planes at right angles to the direction of travel. If, however, this light is passed through a device called a polarizer, only the light waves oscillating in a single plane pass through, while light waves in all other planes are blocked out. This is known as plane-polarized light. A similar effect is achieved in polarized sunglasses or windshields to reduce glare. In the early 1800s, it was discovered that when a beam of plane-polarized light passes through a solution of optical isomers, they rotate the plane of polarization.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 10
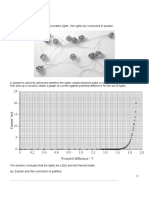
The amount and direction of rotation can be measured with an instrument called a polarimeter as shown in the following figure.
The solution of isomers is placed in the sample tube through which plane polarized light is passed. Rotation of the polarization plane occurs and the light then passes through a second polarizer called the analyser, which has been rotated until the light passes through it. Thus the extent and direction of rotation brought about by the sample can be deduced. In order to compare different solutions, the concentrations of the solutions, the wavelength of light used and the sample path length must all be kept the same. The pioneer of polarimetry was Jean Baptiste Biot (1774-1862), a French physicist and older friend of the famous French bacteriologist Louis Pasteur (1822-1895). Biot showed that some crystals of quartz rotated the plane of polarized light while other crystals rotated it to the same extent in the opposite direction. Later, by showing the same effect in liquids such as turpentine, and in solutions of naturally occurring substances such as sugar, he realized it must be a molecular property and coined the term optical activity. In 1848, Pasteur was working on crystalline salts derived from wine and discovered that while tartaric acid showed optical activity, racemic acid with the same chemical composition did not. He deduced that this was because racemic acid contained an equal mixture of two isomers (such mixtures are now described as racemic). Pasteur saw the huge significance of this. He reasoned that reactions outside the cell always produce an optically inactive mixture whereas biological activity is specific to one isomer. In his later work on the origin of life, this became his guiding distinction between living and inanimate material. Different notations are used to distinguish the two enantiomers of a pair: (+) and (-) refer to the direction in which the plane-polarized light is rotated; (+) for a clockwise direction and (-) for anticlockwise rotation. The lower-case letters d(dextrorotatory) and 1-(Ievorotatory) respectively have traditionally been used as alternatives for this but are becoming obsolete. Confusingly, D- and L- are a different, unrelated notation based on spatial configurations in comparison with the reference molecule glyceraldehyde. This system is widely used in naming many biological molecules such as amino acids and sugars. Other molecules are described by their absolute configuration, using R (rectus) for right or clockwise and S (sinister) for left or counter-clockwise. The rules for determining the absolute configuration are based on atomic number and mass.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 11
Happily, we will not adopt any particular system here and you will not be expected to identify the specific enantiomer in any of these examples. Separate solutions of enantiomers, at the same concentration, rotate plane-polarized light in equal amounts but opposite directions. A racemic mixture does not rotate the light, which is why it is said to be optically inactive. Naturally occurring chiral molecules are optically active, in other words they exist as only one enantiomer. For example, morphine rotates plane-polarized light to the left so is said to be (- ), whereas sucrose rotates plane-polarized light to the right and is said to be (+).
Reactivity with other chiral molecules When a racemic mixture is reacted with a single enantiomer of another chiral compound, the two components of the mixture, the (+) and ( - ) enantiomers, react to produce different products. These products have distinct chemical and physical properties and so can be separated from each other relatively easily. This method of separating the two enantiomers from a racemic mixture is known as resolution. The different reactivity of a pair of enantiomers with another chiral molecule is of particular significance in biological systems because these are chiral environments. An infamous example of the different reactivities of enantiomers occurred in the 1960s when thalidomide was prescribed to pregnant women for morning sickness. One enantiomer is therapeutic but the other produces severe malformations in the fetus. This tragedy largely spearheaded research into processes for the manufacture of a single enantiomer using a chiral catalyst. The process, known as asymmetric synthesis, was developed by three scientists who shared the Nobel Prize in Chemistry in 2001. Other examples of the importance of chirality from biology include the fact that taste buds on the tongue and sense receptors in the nose contain chiral molecules and so interact differently with the different enantiomers. For example, D-amino acids all taste sweet, whereas L-amino acids are often tasteless or bitter. Similarly, we can distinguish between the smells of oranges and lemons due to the presence of different enantiomers of the compound limonene.
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 12
Problems: Answer each problem. For multiple choice questions, include your reasoning in the margins. Which compound can exist as optical isomers? A. CH3CHBrCH3 B. CH2ClCH(OH)CH2CI C. CH3CHBrCOOH D. CH3CCI2CHOH Write the structure of the first alkane in the homologous series to show optical isomerism.
Draw and name the geometric isomers of: (a) pent-2-ene
(b) 2,3-dichlorobut-2-ene.
Which species will show optical activity? A. 1-chloropentane B. 3-chloropentane C. 1-chloro-2-methylpentane D. 2-chloro-2-methylpentane
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 13
(a) (i) The compound C3H6 can react with bromine. Write an equation for this reaction and name the product. State a visible change which accompanies the reaction. (3)
(ii) Give the full structural formula of the product formed in part (a)(i), and identify, by using an asterisk (*), a chiral carbon atom. State what distinctive property a chiral carbon atom gives to a molecule. (2)
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 14
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 15
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 16
(This question is continued on the next page.)
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 17
Chempocalypse Now!
Chapter 13 Stereoisomerism
Page 18
Вам также может понравиться
- Stereoisomerism - Geometric IsomerismДокумент4 страницыStereoisomerism - Geometric IsomerismGopi KupuchittyОценок пока нет
- Stereoisomerism - Wikipedia PDFДокумент18 страницStereoisomerism - Wikipedia PDFChella DuraiОценок пока нет
- Paccon Experimental Physics GuideДокумент215 страницPaccon Experimental Physics GuideDe Silver BettoОценок пока нет
- Medicinal Chemistry—III: Main Lectures Presented at the Third International Symposium on Medicinal ChemistryОт EverandMedicinal Chemistry—III: Main Lectures Presented at the Third International Symposium on Medicinal ChemistryP. PratesiОценок пока нет
- AQA Physics Practicals Apparatus Set Up GuidesДокумент2 страницыAQA Physics Practicals Apparatus Set Up GuidesfoneromОценок пока нет
- Physics: Student Book 2Документ23 страницыPhysics: Student Book 2Tanishka VermaОценок пока нет
- Measurement of Length - Screw Gauge (Physics) Question BankОт EverandMeasurement of Length - Screw Gauge (Physics) Question BankОценок пока нет
- The Principles of Ion-Selective Electrodes and of Membrane TransportОт EverandThe Principles of Ion-Selective Electrodes and of Membrane TransportОценок пока нет
- The Determination of Carboxylic Functional Groups: Monographs in Organic Functional Group AnalysisОт EverandThe Determination of Carboxylic Functional Groups: Monographs in Organic Functional Group AnalysisОценок пока нет
- Stereoisomerism-III Sem B.SCДокумент46 страницStereoisomerism-III Sem B.SCgirishОценок пока нет
- Transition Metal ToxicityОт EverandTransition Metal ToxicityG. W. RichterОценок пока нет
- L2 Che101Документ16 страницL2 Che101Musa Ahammed MahinОценок пока нет
- A2 ChemДокумент81 страницаA2 ChemJana Mohamed100% (1)
- Units and DimensionsДокумент61 страницаUnits and DimensionsPrudhvi RajОценок пока нет
- Y12 Electricty Exam Practice 2Документ14 страницY12 Electricty Exam Practice 2JumanaAsimОценок пока нет
- Chemistry Notes Chap 2 Structure of An AtomДокумент15 страницChemistry Notes Chap 2 Structure of An AtomJo ParkerОценок пока нет
- Chemistry Edexcel As Keywords Unit 1Документ4 страницыChemistry Edexcel As Keywords Unit 1Ashan BopitiyaОценок пока нет
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksОт EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksОценок пока нет
- Simple PendulmДокумент14 страницSimple Pendulmmohit sharmaОценок пока нет
- As and A Level Physics Core Practical 6 SpeeДокумент5 страницAs and A Level Physics Core Practical 6 SpeeHady JawadОценок пока нет
- 04 Chem Review 3Документ36 страниц04 Chem Review 3Khizra Abdul shakoorОценок пока нет
- Summary Notes - Topic 5 Edexcel (A) Biology A-LevelДокумент6 страницSummary Notes - Topic 5 Edexcel (A) Biology A-LevelAhmad MohdОценок пока нет
- Chapter 15: Thermochemistry Key Notes: Fundamentals Aspects Thermochemistry Is AnДокумент11 страницChapter 15: Thermochemistry Key Notes: Fundamentals Aspects Thermochemistry Is AnSarthakОценок пока нет
- Rutherford's Alpha Particle Scattering Experiment Explains Nuclear Model of the AtomДокумент9 страницRutherford's Alpha Particle Scattering Experiment Explains Nuclear Model of the AtomSahil Chawla100% (1)
- Chemical Equilibria Notes PDFДокумент8 страницChemical Equilibria Notes PDFdanielmahsa0% (1)
- Summary Notes - Topic 6 Microbiology, Immunity and Forensics - Edexcel (IAL) Biology A-LevelДокумент11 страницSummary Notes - Topic 6 Microbiology, Immunity and Forensics - Edexcel (IAL) Biology A-LevelsalmaОценок пока нет
- Molecularity of Molecules in ChemistryДокумент13 страницMolecularity of Molecules in ChemistryAhmed HassanОценок пока нет
- Biology Summer Study Transport in PlantsДокумент9 страницBiology Summer Study Transport in PlantsAya100% (1)
- AC Circuit ConceptsДокумент92 страницыAC Circuit ConceptsnajitzaqaroobaОценок пока нет
- Electrochemistry 494 PDFДокумент55 страницElectrochemistry 494 PDFHarsh SaxenaОценок пока нет
- Phy 119 General Physics Practical 20182019Документ18 страницPhy 119 General Physics Practical 20182019MaryjaneОценок пока нет
- Oscillations QuestionsOscillations QuestionsДокумент61 страницаOscillations QuestionsOscillations QuestionsAli SajjadОценок пока нет
- Magnetic MaterialsДокумент18 страницMagnetic MaterialsDinsefa MensurОценок пока нет
- Physics 1922 – 1941: Including Presentation Speeches and Laureates' BiographiesОт EverandPhysics 1922 – 1941: Including Presentation Speeches and Laureates' BiographiesОценок пока нет
- Loudon5ech07sec04 Cyclohexane ConformationsДокумент8 страницLoudon5ech07sec04 Cyclohexane ConformationsF1234TОценок пока нет
- Thermochemistry: - Petrucci, Herring Madura and BissonnetteДокумент49 страницThermochemistry: - Petrucci, Herring Madura and BissonnetteYousif Khalid100% (1)
- Detailed Notes Topic 5 Formulae Equations and Amounts of Substance Edexcel Chemistry A LevelДокумент7 страницDetailed Notes Topic 5 Formulae Equations and Amounts of Substance Edexcel Chemistry A LevelttjjjОценок пока нет
- Pam BlosumДокумент71 страницаPam Blosumrck46100% (1)
- Edexcel IAL As Physics Revision Guide Unit 1AДокумент54 страницыEdexcel IAL As Physics Revision Guide Unit 1ATHE PSYCOОценок пока нет
- Phy SuperpositionДокумент4 страницыPhy Superpositionnewtonian_physicsОценок пока нет
- Boger CourseДокумент477 страницBoger CourseharrypoutreurОценок пока нет
- Modern Physics and Quantum Mechanics Mod-2 PDFДокумент28 страницModern Physics and Quantum Mechanics Mod-2 PDFShreyas SeshadriОценок пока нет
- Edexcel A2 Chemistry Paper 6Документ148 страницEdexcel A2 Chemistry Paper 6AbdulRahman MustafaОценок пока нет
- CH2203 - Spectroscopy of Inorganic CompoundsДокумент6 страницCH2203 - Spectroscopy of Inorganic CompoundsJohnОценок пока нет
- ChB2 Stereoisomerism and ChiralityДокумент23 страницыChB2 Stereoisomerism and Chiralitycassie010890Оценок пока нет
- CIE Physics IGCSE Topic 2 Thermal Physics Summary NotesДокумент5 страницCIE Physics IGCSE Topic 2 Thermal Physics Summary NotesnidhiОценок пока нет
- Empirical and Molecular Formulae WorksheetДокумент3 страницыEmpirical and Molecular Formulae WorksheetJohnclyde Ferry100% (1)
- Moles Equations AtomsДокумент44 страницыMoles Equations AtomsRamesh IyerОценок пока нет
- Understanding Gas LawsДокумент52 страницыUnderstanding Gas LawsLolindah ChinОценок пока нет
- Polymer Analysis TechniquesДокумент100 страницPolymer Analysis Techniquesac.diogo487Оценок пока нет
- 01 StereochemistryДокумент6 страниц01 StereochemistryGundum Bodyz100% (1)
- Goldsure Frekuensi Pemberian (ML) Jumlah (GR) Kalori Protein (GR) 1 100 0 100 3.92Документ9 страницGoldsure Frekuensi Pemberian (ML) Jumlah (GR) Kalori Protein (GR) 1 100 0 100 3.92oktaviana arumОценок пока нет
- Iso 17495-1-1 PDFДокумент23 страницыIso 17495-1-1 PDFjaymin444Оценок пока нет
- MSPL - DuPont KalrezДокумент2 страницыMSPL - DuPont KalrezAnilОценок пока нет
- Lecture Notes ADSORPTIONДокумент15 страницLecture Notes ADSORPTIONtony frankОценок пока нет
- Kamias As Stain RemoverДокумент20 страницKamias As Stain RemoverTrisha Mae Tapawan100% (1)
- Solutions Notes TeacherДокумент4 страницыSolutions Notes TeacherSanaanKhanОценок пока нет
- US20060183879A1Документ6 страницUS20060183879A1julianpellegrini860Оценок пока нет
- SCCP Opinion on Furocoumarins in Cosmetic ProductsДокумент9 страницSCCP Opinion on Furocoumarins in Cosmetic ProductsWisnu Pangarso WibowoОценок пока нет
- Food Chemistry: Tuberosus) Cultivated in EcuadorДокумент8 страницFood Chemistry: Tuberosus) Cultivated in EcuadorChes HauОценок пока нет
- An Improved Synthesis of The Insensitive Energetic Material 3-Amino-5-Nitro - 1,2,4-Triazole (ANTA) in A Simple Single-Step 1-Pot ProcedureДокумент8 страницAn Improved Synthesis of The Insensitive Energetic Material 3-Amino-5-Nitro - 1,2,4-Triazole (ANTA) in A Simple Single-Step 1-Pot ProcedurePatrikОценок пока нет
- ASTM D200 SAE J200 (2) ASTM and SAE Rubber Code Table - enДокумент2 страницыASTM D200 SAE J200 (2) ASTM and SAE Rubber Code Table - enAman Katiyar0% (1)
- Module 10 Physical ScienceДокумент5 страницModule 10 Physical ScienceElixa HernandezОценок пока нет
- Quantum Chemistry Presentation 3Документ36 страницQuantum Chemistry Presentation 3AV Chemistry ClubОценок пока нет
- Test Bank For Brock Biology of Microorganisms 14th Edition Michael T Madigan DownloadДокумент36 страницTest Bank For Brock Biology of Microorganisms 14th Edition Michael T Madigan Downloadautuniteanthesis7y8j100% (42)
- Lecture-11: Study Material Chem-524Документ10 страницLecture-11: Study Material Chem-524Abdul Munaf Ali NasirОценок пока нет
- This Study Resource Was: Answers To Chapter 7Документ6 страницThis Study Resource Was: Answers To Chapter 7Anonymous lDXcsgОценок пока нет
- Kaminsky 2000 PDFДокумент13 страницKaminsky 2000 PDFJaviera Aburto UlloaОценок пока нет
- Push-Out Bond Strength of MTA HP, A New High-Plasticity Calcium Silicate-Based CementДокумент5 страницPush-Out Bond Strength of MTA HP, A New High-Plasticity Calcium Silicate-Based CementCarlos VillavicencioОценок пока нет
- Lab Report 1 CMT659 Determination of Adsorption Isotherm of Acetic Acid On Activated CharcoalДокумент7 страницLab Report 1 CMT659 Determination of Adsorption Isotherm of Acetic Acid On Activated Charcoallina kwikОценок пока нет
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsДокумент51 страницаWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsClaudia TiffanyОценок пока нет
- CAT - PerkinElmer HPLC ConsumablesДокумент44 страницыCAT - PerkinElmer HPLC ConsumablesJosue Daniel MoralesОценок пока нет
- Indonesian Journal of Science & TechnologyДокумент10 страницIndonesian Journal of Science & Technologymuhammad asrunОценок пока нет
- Alkyl Halides & Aryl Halides: (Subjective Problems) (Objective Problems)Документ68 страницAlkyl Halides & Aryl Halides: (Subjective Problems) (Objective Problems)Debayanbasu.juОценок пока нет
- Ureaformaldehyde-Resins (UF Resins) Con ResaltadorДокумент40 страницUreaformaldehyde-Resins (UF Resins) Con ResaltadorIvan Fisgativa VillarragaОценок пока нет
- Active Polymer AP-116 900Документ4 страницыActive Polymer AP-116 900wulalan wulanОценок пока нет
- Sample analysis summaryДокумент1 страницаSample analysis summaryFaizalNurОценок пока нет
- Titration Lab ReportДокумент4 страницыTitration Lab ReportYoonseo (Elin) ChaОценок пока нет
- CONCRETING INSPECTION CHECKLISTДокумент2 страницыCONCRETING INSPECTION CHECKLISTJabinОценок пока нет
- EN Water Treatment DR JuhnkeДокумент11 страницEN Water Treatment DR JuhnkeChwiha LakkОценок пока нет
- General Physics 1: Quarter 2 - Module 5Документ43 страницыGeneral Physics 1: Quarter 2 - Module 5Leica Mariel100% (1)