Академический Документы
Профессиональный Документы
Культура Документы
What is Electropolishing and Why is it Used
Загружено:
gopinath_rgsИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
What is Electropolishing and Why is it Used
Загружено:
gopinath_rgsАвторское право:
Доступные форматы
What is Electropolishing?
1. Electropolishing in an anodic dissolution process in which the =etallic anode surface is smoothed and brightened under optimum conditions of =urrent density and temperature. 2. A method of polishing metal surfaces by applying an electric =urrent through an electrolytic bath in a process that is the reverse of =lating. 3. An electrolytic method of producing simultaneous brightening, =moothing, deburring, cleaning, and passivation on stainless steels. =nbsp;
Why is Electropolishing Used?
Benefits of Electropolishing
1. EP does something for stainless steel which cant be done =ny other way. 2. Simultaneously:
Deburrs Smoothes Brightens Passivates Redefines oxide layer Removes surface contaminants
Electropolish produces =he most spectacular results on 300 series stainless steels. The resulting finish =ften appears bright, shiny, and comparable to the mirror finishes of =E2bright chrome automotive parts. On 400 series stainless steels, the cosmetic =ppearance of the parts is less spectacular, but deburring, cleaning, and passivation are comparable. Solutions are available to electropolish most common =etals. Notable exceptions include cast alloys of zinc, aluminum, brass, bronze, =nd carbon steel. Investment cast stainless steels may also be difficult to electropolish to satisfactory finish unless parts are solution annealed =fter heat treating. In general, only the 200 and 300 series stainless steels, =ertain tool steels, copper, and some single-phase brass alloys can b e =lectropolished to mirror finishes. The principal effects on other types of metal are =eburring, smoothing, improvement of surface finish, and increased adhesion of =lated coatings. Electropolishing produces a combination of properties =hich can be achieved by no other method of surface finishing. Mechanical =rinding, belting, and buffing can produce beautiful mirror-like results on =tainless steel, but the processes are labor intensive and leave the
surface layer =distorted, highly stressed, but do not achieve the bright, lustrous =ppearance obtained by electropolishing. The corrosion resistance of =lectropolished stainless steel exceeds that of standard passivation processes. Electroplating can produce extremely bright finishes, =ut the finish is coating which can chip or wear off. Electroplated surfaces may =lso exhibit hydrogen embrittlement which must be stress-relieved in a =eparate step. Neither passivation nor electroplating can accomplish burr removal. Processes are available for chemical deburring and =rightening of steel and stainless steel, but these methods cannot match the surface =improvement produced by electropolishing. The corrosion resistance =roduced by such processes is decidedly inferior to that produced by =lectropolishing.
ELECTROPOLISHING PROCESS REQUIREMENTS
Electropolishing is =ccomplished in a series of wet processing steps using specially designed tanks, =imilar to electroplating or anodizing. The parts to be polished are mounted on a =ack or jig which is moved from tank to tank. The three major process steps of =he elctropolishing system are: &nb=p; &nbs=; = =nbsp; &=bsp; &n=sp; & nb=p; &nbs=; = =nbsp; &=bsp; &n=sp;
METAL PREPARATION:
&=bsp;
&n=sp;
To remove all oils, lubricants, shop dirt, fingerprints, oxides, =nd other contaminants from the surface. Suitable methods include vapor =egreasing, alkaline and/or acid cleaning, spray washing, abrasive blasting, wire brushing, and other types of mechanical steps.
ELECTROPOLISH:
&=bsp;
&n=sp;
To smooth, brighten, deburr, passivate, stress relieve, improve =urface profile, hygienically clean, reduce friction, increase corrosion =esistance. To remove and recover electropolishing solution.
POST TREATMENT:
&nb=p;
&nbs=;
To remove chemical residues or byproducts of electropolishing and =o assist drying.
Electropolishing of Stainless Steel
Electropolishing is used as = replacement for mechanical finishing, polishing, buffing and mass =inishing. In addition to making a parts surface smoother, it is a more =isible means of brightening, deburring, passivating, stress relieving and otherwise =mproving the physical characteristics of most metals and alloys.
Completely Automated Process Line to Insure Consistent and =epeatable Quality Serving Industries from Aviation to Food Processing to =harmaceutical Small Parts, Large Quantity
What is Electropolishing?
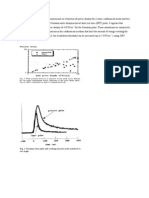
Electropolishing is a process of =emoving metal from a work piece by the passage of electric current while the =ork is submerged in a specially designed electrolyte. The process is =ssentially the reverse of electroplating. In a plating system, metal ions are deposited =rom the solution onto the work piece; in an electropolishing system, the =ork piece itself is dissolved, adding metal ions to the solution. The work =iece is connected to the positive (or anodic) terminal, while the negative =cathodic) terminal is connected to a suitable conductor. Both positive and =egative terminals are submerged in the electrolyte, forming a complete =lectrical circuit. The current applied is direct (DC) current. The quantity =f metal removed from the work piece is proportional to the mount of =urrent applied and the time. In the course of electropolishing, burrs and other =projections become very high current density areas and are rapidly =issolved. The work piece is manipulated to control the amount of metal removal so =hat dimensional tolerances are maintained. In the case of stainless =teel alloys, an important effect is caused by differences in the rates of =emoval of the components of alloy. Iron and nickel atoms are more easily extracted =rom the crystal lattice than are chromium atoms. The electropolishing =rocess removes the nickel and the iron preferentially, leaving a surface rich =n chromium. This phenomenon imparts the important property of =E2passivation to electropolished surfaces.
Reasons for Electropolishing
Many products are electropolished =imply to remove fine burrs from stampings, machined surfaces, perforated sheet, =nd many other types of products. For large burrs, some mechanical grinding may =e required prior to electropolishing.
Electropolishing produces
excellent decorative =inishes for a wide range of stainless steel automotive, appliance, and household =roducts. The surface produced combines attractive appearance with improved =orrosion resistance. Electropolishing improves corrosion resistance by =educing surface area, eliminating occlusions, reducing free iron, and producing = passivating film of a corrosion resistant chromium oxide. The special =roperties of the oxide layer are of great importance in semiconductor and =harmaceutical applications requiring a clean, sanitary surface with little or no =endency to react with a liquid of gaseous chemical environment. Some steel =roducts, such as textile rolls, are electropolished prior to being plated with =ard chrome. The smoothing and leveling effect of the electropolishing causes =he chromium to deposit in a more regular fashion, improving the properties =f the plate and strengthening the bond with the substrate steel. Electropolishing also finds application in products =equiring smooth, low-friction operation. Products such as powdered =harmaceuticals, dyes, and other dry chemicals are processed with minimal losses due to the low =friction surface produced. A major application for =lectropolishing is in equipment for the manufacture and packaging of food, beverage, and pharmaceutical products. The processing equipment is regularly treated =ith clean-in-place chemicals to remove traces of product =etween batches and to maintain sanitary conditions. Electropolished stainless steel offers a =urface which is readily cleaned by such processes.
Вам также может понравиться
- ANODIZING: ELECTROLYTIC PROCESS INCREASES ALUMINUM OXIDE LAYERДокумент4 страницыANODIZING: ELECTROLYTIC PROCESS INCREASES ALUMINUM OXIDE LAYERShubham JainОценок пока нет
- 0001 CatДокумент108 страниц0001 CatJorge CabreraОценок пока нет
- Black Oxide MIL DTL 13924DДокумент11 страницBlack Oxide MIL DTL 13924DadamhomeОценок пока нет
- Electroplating and Electroless Plating Corrosion EngineeringДокумент10 страницElectroplating and Electroless Plating Corrosion Engineeringmm11_nedОценок пока нет
- The Pentester BlueprintДокумент27 страницThe Pentester Blueprintjames smith100% (1)
- Chrome PlatingДокумент14 страницChrome Platingsonnu151Оценок пока нет
- Black PassivationДокумент12 страницBlack PassivationZineb100% (1)
- AnodizingДокумент9 страницAnodizingalphadingОценок пока нет
- Chrome PlatingДокумент11 страницChrome PlatingMahesh Babu100% (1)
- Anodic Oxidation of Aluminium and Its Alloys: The Pergamon Materials Engineering Practice SeriesОт EverandAnodic Oxidation of Aluminium and Its Alloys: The Pergamon Materials Engineering Practice SeriesРейтинг: 5 из 5 звезд5/5 (1)
- Electropolishing PDFДокумент30 страницElectropolishing PDFAnonymous uL3JlWfh100% (2)
- Surface Finish Basics For Stainless Steel Rev 1Документ30 страницSurface Finish Basics For Stainless Steel Rev 1Nitasana SilapornОценок пока нет
- An Overview of Hard Chromium Plating Using Trivalent ChromiumДокумент9 страницAn Overview of Hard Chromium Plating Using Trivalent ChromiumthuronОценок пока нет
- Black OxideДокумент2 страницыBlack OxideGerman ToledoОценок пока нет
- Navajo Hearing ProgramДокумент3 страницыNavajo Hearing Programjamesmith100000% (1)
- The Motive Journal (3rd Edition)Документ42 страницыThe Motive Journal (3rd Edition)Shubham Sharma0% (1)
- S6MT 1Q w1 3 MELC1 SLM MIXTURES FinalCopy09082020Документ26 страницS6MT 1Q w1 3 MELC1 SLM MIXTURES FinalCopy09082020Rona Dindang100% (1)
- Passivation Treatment of Stainless Steel AcomДокумент10 страницPassivation Treatment of Stainless Steel Acomvijayarangam1984Оценок пока нет
- Chromium PlatingДокумент22 страницыChromium PlatingKodeboyina ChandramohanОценок пока нет
- 2010 Section IX Etching ProcessesДокумент1 страница2010 Section IX Etching ProcessesSARSAN NDTОценок пока нет
- ElectropolishingДокумент6 страницElectropolishingnagurvali65Оценок пока нет
- Nitinol Af Testing Methods and ConsiderationsДокумент5 страницNitinol Af Testing Methods and ConsiderationsTodd DicksonОценок пока нет
- Hard Chrome PlatingДокумент2 страницыHard Chrome PlatingGuru SamyОценок пока нет
- CrO3 Alternatives in Decorative and Functional Plating PDFДокумент22 страницыCrO3 Alternatives in Decorative and Functional Plating PDFLukeОценок пока нет
- Principles of Metal Surface Treatment and Protection: Pergamon International Library of Science, Technology, Engineering and Social Studies: International Series on Materials Science and TechnologyОт EverandPrinciples of Metal Surface Treatment and Protection: Pergamon International Library of Science, Technology, Engineering and Social Studies: International Series on Materials Science and TechnologyОценок пока нет
- Welding Metallurgy and Weldability of Nickel-Base AlloysОт EverandWelding Metallurgy and Weldability of Nickel-Base AlloysРейтинг: 5 из 5 звезд5/5 (1)
- Tin Plating ProcessДокумент3 страницыTin Plating Processkrishy76100% (1)
- Astm E1558.24503Документ13 страницAstm E1558.24503Juan Shevchenko100% (1)
- Hull Cell Yamamoto MS PDFДокумент16 страницHull Cell Yamamoto MS PDFHaydee VОценок пока нет
- Electropolishing WorkbookДокумент16 страницElectropolishing WorkbookAhmet BozgeyikОценок пока нет
- Electrochemical PolishingДокумент10 страницElectrochemical Polishingeasch75Оценок пока нет
- Role of phosphoric acid in electropolishing stainless steelДокумент1 страницаRole of phosphoric acid in electropolishing stainless steelParveen KohliОценок пока нет
- Electropolishing Using Ionic Liquids: A Greener AlternativeДокумент26 страницElectropolishing Using Ionic Liquids: A Greener AlternativetetirichieОценок пока нет
- SCAT Chart - Systematic Cause Analysis Technique - SCAT ChartДокумент6 страницSCAT Chart - Systematic Cause Analysis Technique - SCAT ChartSalman Alfarisi100% (1)
- Electro-Polishing: Prepared byДокумент44 страницыElectro-Polishing: Prepared byMahesh Kumar100% (3)
- Supplier Assessment Report-Shenzhen Illuman Photoelectronic Co., LTDДокумент29 страницSupplier Assessment Report-Shenzhen Illuman Photoelectronic Co., LTDAdam Andrew OngОценок пока нет
- Astm b912 - 00 Passivation of Stainless Steels Using Electropolishing PDFДокумент4 страницыAstm b912 - 00 Passivation of Stainless Steels Using Electropolishing PDFcaballerolangОценок пока нет
- Astm b650 Proceso de PlatingДокумент5 страницAstm b650 Proceso de PlatingALEXANDRA TALAMANTESОценок пока нет
- Chromate Conversion Coating and Alternatives As Corrosion-Resistant Treatments For Metal Parts v1Документ17 страницChromate Conversion Coating and Alternatives As Corrosion-Resistant Treatments For Metal Parts v1Ivy LiОценок пока нет
- Environmentally Friendly Zirconium Oxide PretreatmentДокумент76 страницEnvironmentally Friendly Zirconium Oxide Pretreatmentalecandro_90Оценок пока нет
- Corrosion Testing for Metal Finishing: Institute of Metal FinishingОт EverandCorrosion Testing for Metal Finishing: Institute of Metal FinishingОценок пока нет
- Adult Congenital Heart Disease Board ReviewДокумент76 страницAdult Congenital Heart Disease Board ReviewOQAB13Оценок пока нет
- Advances in Surface Treatments: Technology — Applications — EffectsОт EverandAdvances in Surface Treatments: Technology — Applications — EffectsОценок пока нет
- ATOTECH - EcoTri - Bright Zinc Plating - Hexavalent Chrome FreeДокумент4 страницыATOTECH - EcoTri - Bright Zinc Plating - Hexavalent Chrome FreeWK Sinn100% (1)
- DacrometДокумент6 страницDacrometdavideОценок пока нет
- TALAT Lecture 5201: Aluminium Surface PretreatmentДокумент12 страницTALAT Lecture 5201: Aluminium Surface PretreatmentCORE Materials100% (1)
- NASA Electropolishing Process for Corrosion-Resistant SteelДокумент7 страницNASA Electropolishing Process for Corrosion-Resistant SteelHenryОценок пока нет
- Data Sheet AluminaДокумент10 страницData Sheet AluminaXin EnОценок пока нет
- Chrome Plating and Anodizing Operations GuideДокумент6 страницChrome Plating and Anodizing Operations GuidePuguh Cahpordjo BaeОценок пока нет
- Modern Electroplating Fourth Edition Edited by M SДокумент2 страницыModern Electroplating Fourth Edition Edited by M Smiguelin91690% (1)
- Heavy Phosphate Coatings SpecificationДокумент17 страницHeavy Phosphate Coatings Specificationrobert_in_ar100% (1)
- Chromate Conversion Coatings On Aluminium - Influences of AlloyingДокумент16 страницChromate Conversion Coatings On Aluminium - Influences of AlloyingDaniel Alfonso Moreno VerbelОценок пока нет
- New Galvanizing TechnologyДокумент4 страницыNew Galvanizing Technologywmaddoxmec100% (1)
- Technical Data Sheet: Trichrome HB 1700 TДокумент3 страницыTechnical Data Sheet: Trichrome HB 1700 TLuuThiThuyDuong100% (1)
- Decorative Chromium PlatingДокумент8 страницDecorative Chromium Platingcauthon82Оценок пока нет
- Metal Finishing NovDec2012Документ60 страницMetal Finishing NovDec2012anacrisst100% (1)
- ISO9000:2000 Certified Chem Film Coating ServicesДокумент1 страницаISO9000:2000 Certified Chem Film Coating ServicesShahid Ahmed.cОценок пока нет
- Standard Specification For: Designation: B6 13Документ3 страницыStandard Specification For: Designation: B6 13Ahmed BilalОценок пока нет
- Anodizing Aluminum and Microstructure of Steel SprocketДокумент16 страницAnodizing Aluminum and Microstructure of Steel SprocketIlhamChaniefОценок пока нет
- Mil DTF 5541fДокумент12 страницMil DTF 5541fMarcos PerezОценок пока нет
- Chromic Acid Lanxess-MSDS PDFДокумент10 страницChromic Acid Lanxess-MSDS PDFyuk ming wongОценок пока нет
- Blackening Processes For ZincДокумент13 страницBlackening Processes For Zincvasudev_nОценок пока нет
- DG Gsa VatДокумент2 страницыDG Gsa Vatgopinath_rgsОценок пока нет
- Blood Group TestДокумент1 страницаBlood Group Testgopinath_rgsОценок пока нет
- Courses of Study and Scheme of Assessment Be Production Engineering (Документ8 страницCourses of Study and Scheme of Assessment Be Production Engineering (gopinath_rgsОценок пока нет
- Tool Consumption Value 23122008Документ1 страницаTool Consumption Value 23122008gopinath_rgsОценок пока нет
- About PSG Tech & CADD Lab: About The MCAD Applications:: Pro/Engineer WildfireДокумент2 страницыAbout PSG Tech & CADD Lab: About The MCAD Applications:: Pro/Engineer Wildfiregopinath_rgsОценок пока нет
- Tool Consumption Value 23122008Документ1 страницаTool Consumption Value 23122008gopinath_rgsОценок пока нет
- Decmonth Daily ConsumptionДокумент12 страницDecmonth Daily Consumptiongopinath_rgsОценок пока нет
- Development IdeaДокумент1 страницаDevelopment Ideagopinath_rgsОценок пока нет
- PSG CourseДокумент2 страницыPSG Coursegopinath_rgsОценок пока нет
- DevelopmentsДокумент3 страницыDevelopmentsgopinath_rgsОценок пока нет
- Q7 User ManualДокумент34 страницыQ7 User ManualJaspal SinghОценок пока нет
- ĐỀ THI THU TNTHPT SỐ 17Документ4 страницыĐỀ THI THU TNTHPT SỐ 17Nguyên Hà NguyễnОценок пока нет
- Catálogo SEDIVERДокумент32 страницыCatálogo SEDIVEREnver Rojas DiazОценок пока нет
- Yanagiba Sharpening: Everything You Need To KnowДокумент16 страницYanagiba Sharpening: Everything You Need To KnowT ChenОценок пока нет
- RESEARCH PROPOSAL-Final AfraaaazzzzzzzzzДокумент13 страницRESEARCH PROPOSAL-Final AfraaaazzzzzzzzzRizwan Abdul Maalik50% (2)
- Bio ViberДокумент7 страницBio ViberMarco BuntОценок пока нет
- App 17 Venmyn Rand Summary PDFДокумент43 страницыApp 17 Venmyn Rand Summary PDF2fercepolОценок пока нет
- The Congressional Committee and Philippine Policymaking: The Case of The Anti-Rape Law - Myrna LavidesДокумент29 страницThe Congressional Committee and Philippine Policymaking: The Case of The Anti-Rape Law - Myrna LavidesmarielkuaОценок пока нет
- Guide To Admissions 2024-25Документ159 страницGuide To Admissions 2024-25imayushx.inОценок пока нет
- Fluvial Erosion Processes ExplainedДокумент20 страницFluvial Erosion Processes ExplainedPARAN, DIOSCURAОценок пока нет
- Atomic and Molecular PhysicsДокумент28 страницAtomic and Molecular PhysicsAvinash GuptaОценок пока нет
- EMI InstructionsДокумент2 страницыEMI InstructionsAKSHAY ANANDОценок пока нет
- Variable Displacement Engines: The Magic of Cylinder DeactivationДокумент3 страницыVariable Displacement Engines: The Magic of Cylinder DeactivationdinuОценок пока нет
- Buhos SummaryДокумент1 страницаBuhos Summaryclarissa abigail mandocdocОценок пока нет
- Presente Continuo Present ContinuosДокумент4 страницыPresente Continuo Present ContinuosClaudio AntonioОценок пока нет
- EDSP Quantitative and Qualitative FormДокумент2 страницыEDSP Quantitative and Qualitative FormTalal SultanОценок пока нет
- Regional Ecology Test ScoringДокумент14 страницRegional Ecology Test Scoringaisyah Wardah201Оценок пока нет
- Power Systems-III Ditital NotesДокумент102 страницыPower Systems-III Ditital NotesSimranОценок пока нет
- Chambal Cable Stayed Bridge Connecting ShoresДокумент6 страницChambal Cable Stayed Bridge Connecting Shoresafzal taiОценок пока нет
- Density of Aggregates: ObjectivesДокумент4 страницыDensity of Aggregates: ObjectivesKit Gerald EliasОценок пока нет
- 4 6051111060339957657Документ361 страница4 6051111060339957657Oviedo OviedoОценок пока нет
- EFPSДокумент8 страницEFPSBryan Joshua VillarОценок пока нет
- Abdul Khaliq - Good Governance (GG)Документ15 страницAbdul Khaliq - Good Governance (GG)Toorialai AminОценок пока нет