Академический Документы
Профессиональный Документы
Культура Документы
Cystoid Macular Edema
Загружено:
Agnes Triana BasjaИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Cystoid Macular Edema
Загружено:
Agnes Triana BasjaАвторское право:
Доступные форматы
CYSTOID MACULAR EDEMA
ELLEN N. YU, M.D. CASE REPORT
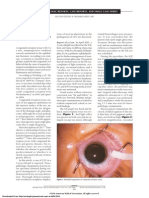
A 41 year old white female presented with a history of recurrent anterior uveitis. The patient was diagnosed with anterior uveitis eighteen months prior to presentation when she consulted her local ophthalmologist for right eye redness, slight pain and blurring of vision. The uveitis resolved with topical steroids, but recurred twice over the past year and a half. There were no associated systemic symptoms. Past medical history and family medical history were unremarkable. On examination, visual acuities were 20/100 on the right and 20/20 on the left. Slit lamp exam of the right eye showed white and quiet conjunctiva, few fine keratic precipitates on the posterior corneal surface and +1/2 cells in the anterior chamber. There was no synechiae or cataract. Slit lamp exam of the left eye was normal. Dilated eye exam of the right eye showed clear vitreous, cup disc ratio of 0.3, no retinal lesions were noted except for a dull foveal reflex (Figure 1). The left fundus was normal. Fluorescein angiogram was done (figures 2 & 3) which revealed the presence of cystoid macular edema. Transeptal steroid injection was administered and oral non-steroidal antiinflammatory drug was initiated for the macular edema. Systemic work-up was negative except for HLA-B27 positivity.
Figure 1. Color photo of right fundus showing dull foveal reflex.
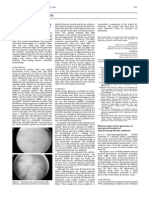
Figure 2. Early phase of fluorescein angiogram of the right eye.
Figure 3. Late phase of fluorescein angiogram of the right eye showing dye leakage in a petalloid pattern at the macular area. INTRODUCTION The macroscopic changes of cystoid macular edema (CME) was first described by Irvine in 1953. A loss of the foveolar reflex in the macula was noted in a patient with decreased visual acuity associated with prolapse of vitreous in the anterior chamber after intracapsular cataract extraction (ICCE). Since that time, macular edema has been identified as a common cause of decreased vision in many ophthalmic diseases. In fact, it is the most common macular alteration associated with uveitis. Although it may occur in any type of ocular inflammation, the types of uveitis most commonly associated with macular edema are: pars planitis, iridocyclitis, birdshot retinochoroidopathy, sarcoid uveitis and HLAB27 uveitis PATHOPHYSIOLOGY Macular edema is caused by disruption in the normal permeability barrier of the retina, the blood-retinal barrier (BRB). The BRB is responsible for restricting movement of plasma constituents into the retina and in maintaining homeostasis. The BRB has 2 components: the inner BRB is composed of the endothelial cells of the retinal blood vessels; the outer BRB is composed of the tight junctions between retinal pigment epithelial (RPE) cells.
When the BRB is disrupted, the volume of the extracellular space of the retina expands due to unrestricted entry of protein and water from plasma. Why the macula is predisposed to accumulation of fluid causing cystoid macular edema is not known. Inflammation is among the factors implicated in BRB breakdown. Other factors include metabolic alterations (diabetes, retinitis pigmentosa), ischemia (severe hypertension, toxemia of pregnancy, collagen vascular disease, disseminated intravascular coagulopathy), increased capillary pressure (venous occlusive disease, systemic hypertension), severe ocular hypotension, mechanical forces (epiretinal membrane), and toxicity (epinephrine, betaxolol, latanoprost). In ocular inflammation, increased production of inflammatory mediators such as prostaglandins lead to increased permeability of the parafoveal capillaries and exudation in the macular area. Non-steroidal anti-inflammatory drugs (NSAIDs) and steroids are thus utilized to target the arachidonic acid pathway in prostaglandin synthesis. The following soluble factors have also been implicated in promoting BRB breakdown leading to macular edema: adenosine, prostaglandin E1 (PGE1), tumor necrosis factor a (TNF a), interleukin-1 b (IL-1 b), vascular endothelial growth factor(VEGF), and vasoactive peptides such as bradykinins or kallikreines. When given intravitreally, these mediators have been noted to cause morphological and functional opening of the retinal vascular endothelium (RVE) tight junctions and upregulation of vesicle-mediated transport across the RVE. The effect of these mediators in the outer BRB is less clear. HISTOPATHOLOGY Light microscopy has shown the presence of fluid accumulation in intraretinal cysts in the inner nuclear and outer plexiform layers of the retina. The cysts are located around the fovea producing a petalloid pattern. With increased severity and chronicity, deeper and larger cysts were noted. Whether these represent smaller cysts that have coalesce has not been mentioned.
Other researchers believe that disruption of muller cell function by ischemia and other factors lead to the development of CME and that the cystic spaces observed were swollen Muller cells The accumulation of fluid in the outer plexiform layer is said to be a late phenomena following breakdown of the Muller cells. However, some authors argue that these findings may be secondary to ischemic changes after cessation of blood flow to the retina during specimen colleciton. Macular changes may be reversible, and restoration of vascular permeability may occur after resolution of the edema. However, after chronic edema, retinal thinning with photoreceptor damage and progressive fibrosis may occur. The length of time required to produce permanent damage is not known. DIAGNOSIS The patient may complain of decreased visual acuity, metamorphopsia and micropsia. To document these, visual acuity testing and amsler grid should be performed. Under the slit lamp using a 78- or 90- diopter aspheric lens, areas with thickening or cystic accumulation of fluid may be detected. Indirect ophthalmoscopy can be utilized to visualize whether there are other areas of thickening or when evaluation is difficult due to hazy media and/or cataract. When changes are very subtle, fluorescein angiography is an important tool in the diagnosis of CME.
FLUORESCEIN ANGIOGRAM Fluorescein angiogram (FA) will show progressive hyperfluorescent leakage of dye in the macular area with late accumulation in the parafoveal cystic spaces resulting in the characteristic petalloid pattern (Figure 3). Studies have shown a poor correlation between dye leakage and visual acuity. In some patients, leakage can occur without decrease in vision. FA is not helpful in predicting the present VA nor the ultimate outcome of therapy. OPTICAL COHERENCE TOMOGRAPHY In optical coherence tomography (OCT), a laser slit beam is projected into the retina and a high-resolution cross-section image is obtained. Localized accumulation of fluid can be seen with increase in retinal thickness. Studies have compared OCT and FA with regards to detection of macular edema and macular thickening has been shown to correlate better with visual acuity. Moreover, OCT has been shown to detect macular thickening even before any angiographic evidence of CME. Thus OCT seems to be a very promising tool. TREATMENT Currently, there is still no accepted treatment modality for uveitis-associated CME. This is due to the fact that there has been no success in producing macular edema experimentally in animals. Data has been based on clinical studies, and many agents are used with variable response. Since there is a constant release of inflammatory mediators when there is active uveitis, the first and most important step is to control the uveitis. (1) Corticosteroids. Steroids have been used to treat uveitis-associated cystoid macular edema since studies have shown that inflammatory mediators lead to increased vascular permeability. Steroids may be given topically, by periocular injection, or orally. Topical steroids can be given to aphakics where it has been shown to reach the posterior pole. Periocular injections can produce high levels in the posterior segment of the eye with a lower risk of systemic side effects than oral steroids. (2) Non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs also affect prostaglandin synthesis and may be given topically or orally. Whether steroids and NSAIDs affect macular edema other than prostaglandin synthesis i.e. whether they act directly on cells, or whether they help them in pumping out fluid more efficiently, is not known. (3) Carbonic anhydrase inhibitors (CAIs). CAIs increase the active transport of fluid from the retina to the choroids by pumps in the pigment epithelial cells . However, recurrence of edema after discontinuation has been reported. (4) Hyperbaric oxygen, vitrectomy and laser photocoagulation. These treatment modalities are still controversial at this point. Mechanism of action of hyberbaric oxygen in improving visual acuity in CME is not known. PROGNOSIS CME requires many months of treatment. It may disappear with resolution of the uveitis. However, even with complete resolution of the inflammation, macular changes secondary to chronic CME may lead to permanent visual loss. Visual acuity improvement is more commonly seen in patients with CME of less than 6 months duration. REFERENCES 1. Irvine SR. A newly defined vitreous syndrome following cataract surgery. Am J Ophthalmol 1953; 36(5): 599-619.
2. Gass JDM, Norton EWD. Cystoid macular edema and papilledema following cataract extraction. Arch Ophthalmol 1966; 76(11):646-661. 3. Nussenblatt RB. Macular alterations secondary to intraocular inflammatory disease. Ophthalmology July 1986; 93(7):984-988. 4. Dick JSB. Macular Edema. Int Ophthalmol Clin 1999 Fall; 39 (4): 1-18. edema.
5. Drews RC. The present understanding of cystoid macular Trans Ophthalmol Soc UK 1985; 104: 744-747.
6. Cunha-Vaz JG, Travassos A. Breakdown of the Blood-Retinal Barriers and cystoid macular edema. Surv Ophthalmol May 1984; 28 (supplement) 485-492. 7. Neufeld AH, Sears ML. Prostaglandins and the eye. Prostaglandins 1973; 4:157-168. 8. Vinores SA et al. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol 1999; 97: 13-24. 9. Tso M. Pathological study of cystoid macular edema. Trans Ophthalmol Soc UK 1980; 100:408-413. 10. Tso M. Pathology and pathogenesis of cystoid macular edema. Ophthalmologica 1981; 1983: 46-54. 11. Tso M. Pathology of cystoid macular edema. Ophthalmology 1982; 89: 902-915. 12. Gass JDM. Stereoscopic atlas of macular diseases 3rd ed. St Louis: Mosby 1987: 374. 13. Varano M et al. New diagnostic tool for macular edema. Doc Ophthalmol 1999; 97: 373-379. 14. Antcliff RJ. Comparison between optical coherence tomography and fundus fluorescein angiography for the detection of cystoid macular edema in patients with uveitis. Ophthalmology Mar 2000; 107 (3): 593-599. 15. Hee MR et al. Quantitative Assessment of macular edema with optical coherence tomography. Arch Ophthalmol Aug 1995; 113: 1019-1029. 16. Nussenblatt RB, Kaufman SC, Palestine AG et al. Macular thickening and visual acuity: measurement in patients with cystoid macular edema. Ophthalmology 1987; 94:11341139. 17. Rojas B et al. Medical treatment of macular edema in patients with uveitis. Doc Ophthalmol 1999; 97: 399-407. 18. Wolfensberger TJ, Herbort CP. Treatment of cystoid macular edema with non-steroidal anti-inflammatory drugs and corticosteroids. Doc Ophthalmol 1999; 97: 381-386. 19. Jennings T, Rusin MM, Tessler HH, Cunha-Vaz JG. Posterior sub-Tenons injections of corticosteroids in uveitis patients with cystoid macular edema. Jpn J Ophthalmol 1988; 32:385-391. 20. Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol 1999; 97 (3-4): 387-97. 21. Suttorp-Schulten MSA et al. Macular grid photocoagulation in uveitis. Br J Ophthalmol Sep 1995; 79 (9): 821-824. 22. Butler CFK. Diving and Hyperbaric Ophthalmology. Surv Ophthalmol 1995; 39 (5): 347366.
Вам также может понравиться
- Complications in UveitisОт EverandComplications in UveitisFrancesco PichiОценок пока нет
- V36N4p293 PDFДокумент5 страницV36N4p293 PDFsiscaОценок пока нет
- Management of Macular HemorrhageОт EverandManagement of Macular HemorrhageLars-Olof HattenbachОценок пока нет
- Toophtj 5Документ7 страницToophtj 5desyОценок пока нет
- 0410RT Feature BennettДокумент4 страницы0410RT Feature BennettRaissaОценок пока нет
- Uveitis GlaukomaДокумент13 страницUveitis GlaukomaDede FatmawatiОценок пока нет
- Vitrectomy Results in Proliferative Diabetic RetinopathyДокумент3 страницыVitrectomy Results in Proliferative Diabetic RetinopathyRohamonangan TheresiaОценок пока нет
- Non-Ischemic Central Retinal Vein OcclusionДокумент6 страницNon-Ischemic Central Retinal Vein OcclusionMahmoud Ahmed MahmoudОценок пока нет
- Vitreous HemorrhageДокумент7 страницVitreous HemorrhageindahОценок пока нет
- Exudative Retinopathy of Adults A Late S PDFДокумент5 страницExudative Retinopathy of Adults A Late S PDFInna BujorОценок пока нет
- Clinicopathologic Reports, Case Reports, and Small Case SeriesДокумент3 страницыClinicopathologic Reports, Case Reports, and Small Case SeriesDiaz RandanilОценок пока нет
- Cytokines Associated With Hemorrhage in Proliferative Diabetic RetinopathyДокумент9 страницCytokines Associated With Hemorrhage in Proliferative Diabetic RetinopathyQabil GilbranОценок пока нет
- A Study On Effectiveness of Intravit Real Ranibizumab For Management of Different Retinal Vascular DisordersДокумент12 страницA Study On Effectiveness of Intravit Real Ranibizumab For Management of Different Retinal Vascular DisordersInternational Journal of Innovative Science and Research TechnologyОценок пока нет
- Diagnosis and Management of Malignant GlaucomaДокумент3 страницыDiagnosis and Management of Malignant GlaucomaYazmin Peñaloza RoaОценок пока нет
- Prabhakar 1Документ4 страницыPrabhakar 1ChristaGisellaPirsouwNoyaОценок пока нет
- 10 1016@j Survophthal 2020 02 004Документ17 страниц10 1016@j Survophthal 2020 02 004AlexОценок пока нет
- Letters To The EditorДокумент8 страницLetters To The EditorGheavita Chandra DewiОценок пока нет
- Is Episcleritis Associated To Glaucoma?Документ3 страницыIs Episcleritis Associated To Glaucoma?Alvin JulianОценок пока нет
- Retina AnswersДокумент5 страницRetina AnswersKhushal Khan KakarОценок пока нет
- Tuberculous Posterior Scleritis Case ReportДокумент6 страницTuberculous Posterior Scleritis Case ReportInayatul muthmainnahОценок пока нет
- Neovascular GlaucomaДокумент4 страницыNeovascular GlaucomaMahmoud ElsaidОценок пока нет
- Fixed and Dilated Pupil Case Report Following Cataract SurgeryДокумент6 страницFixed and Dilated Pupil Case Report Following Cataract SurgeryAi HidayatОценок пока нет
- Retinal LaserДокумент7 страницRetinal LaserSandra BabuОценок пока нет
- Mcculley 2012Документ11 страницMcculley 2012Luisa Mayta PitmanОценок пока нет
- GlaucomaДокумент18 страницGlaucomaOncología CdsОценок пока нет
- Retinal Tear: An Unusual Complication of Ocular ToxoplasmosisДокумент5 страницRetinal Tear: An Unusual Complication of Ocular ToxoplasmosisMuhammad Irfan FaizОценок пока нет
- Central Serous Chorioretinopathy: An Update On Pathogenesis and TreatmentДокумент14 страницCentral Serous Chorioretinopathy: An Update On Pathogenesis and TreatmentMuhammad Bayu Zohari HutagalungОценок пока нет
- Retninal Vein OcclusionДокумент10 страницRetninal Vein OcclusionuhibОценок пока нет
- Primary Retinal DetachmentДокумент9 страницPrimary Retinal DetachmentMarviane ThenОценок пока нет
- Primary Retinal Detachment: Clinical PracticeДокумент9 страницPrimary Retinal Detachment: Clinical PracticeAdita DitaОценок пока нет
- Central Serous Retinopathy: Michael Colucciello, MDДокумент9 страницCentral Serous Retinopathy: Michael Colucciello, MDRani Henty NovitaОценок пока нет
- Observations On Time Course Changes of The CherryДокумент16 страницObservations On Time Course Changes of The CherryAndi Tiara S. AdamОценок пока нет
- Uveitis 2Документ9 страницUveitis 2srihandayaniakbarОценок пока нет
- MainДокумент7 страницMainiSee ClinicОценок пока нет
- Treatment of Macular EdemaДокумент48 страницTreatment of Macular EdemaPushpanjali RamtekeОценок пока нет
- Ocular Ischemic Syndrome Survey OphthalmologyДокумент33 страницыOcular Ischemic Syndrome Survey OphthalmologyamaiacОценок пока нет
- Bodh 2011Документ8 страницBodh 2011syramadhantiОценок пока нет
- Makrufa NWДокумент6 страницMakrufa NWPujiastutiОценок пока нет
- Risk Factors and Visual Effects of Cystoid Macular Edema After Cataract SurgeryДокумент6 страницRisk Factors and Visual Effects of Cystoid Macular Edema After Cataract SurgeryB OriestoОценок пока нет
- Senile Cataract Clinical Presentation and CausesДокумент4 страницыSenile Cataract Clinical Presentation and CausesAhmad FahroziОценок пока нет
- Senile Cataract Clinical Presentation and CausesДокумент4 страницыSenile Cataract Clinical Presentation and CausesAhmad Fahrozi100% (1)
- Vascular Abnormalities in Uveitis: SciencedirectДокумент15 страницVascular Abnormalities in Uveitis: Sciencedirect2456708893Оценок пока нет
- New 5-FU option for epithelial downgrowthДокумент4 страницыNew 5-FU option for epithelial downgrowthDelila MaharaniОценок пока нет
- M J Opht 1 1 004Документ7 страницM J Opht 1 1 004Pra BowoОценок пока нет
- Senile Cataract (Age-Related Cataract) - Practice Essentials, Background, PathophysiologyДокумент5 страницSenile Cataract (Age-Related Cataract) - Practice Essentials, Background, PathophysiologyAhmad FahroziОценок пока нет
- Ocular in Ammatory DiseasesДокумент5 страницOcular in Ammatory DiseasesCharlotteGraceNusiferaОценок пока нет
- Keratoconus: Picture 1Документ6 страницKeratoconus: Picture 1BuiyiWongОценок пока нет
- Keratoconus: Picture 1Документ6 страницKeratoconus: Picture 1BuiyiWongОценок пока нет
- Primary Open-Angle Glaucoma: SeminarДокумент10 страницPrimary Open-Angle Glaucoma: SeminarFebry LuthunananaОценок пока нет
- Vaskulitis ChorioretinopathyДокумент10 страницVaskulitis ChorioretinopathydrheriОценок пока нет
- Glaucoma OverviewДокумент33 страницыGlaucoma Overviewc/risaaq yuusuf ColoowОценок пока нет
- Spontaneous Association of Macular Hole and Choroidal Neovascularization: A Case ReportДокумент4 страницыSpontaneous Association of Macular Hole and Choroidal Neovascularization: A Case ReportIJAR JOURNALОценок пока нет
- Pi Is 0161642022006923Документ12 страницPi Is 0161642022006923Anca Florina GaceaОценок пока нет
- Current Management of Diabetic MaculopathyДокумент8 страницCurrent Management of Diabetic MaculopathyraniОценок пока нет
- 1 s2.0 S0002939412000669 MainДокумент1 страница1 s2.0 S0002939412000669 MainCupris23Оценок пока нет
- Ignacio - 6TH Paper SGD OphthaДокумент4 страницыIgnacio - 6TH Paper SGD OphthaRichelle IgnacioОценок пока нет
- Senile Cataract (Age-Related Cataract) : Practice Essentials, Background, PathophysiologyДокумент6 страницSenile Cataract (Age-Related Cataract) : Practice Essentials, Background, PathophysiologyadliahghaisaniОценок пока нет
- Injeksi Intravitreal Bevacizumab Dan Laser Fotokoagulasi Pada Branch RetinalДокумент8 страницInjeksi Intravitreal Bevacizumab Dan Laser Fotokoagulasi Pada Branch RetinalDwi MasrokhahОценок пока нет
- Pemicu 1 Pengindraan c2Документ113 страницPemicu 1 Pengindraan c2CcОценок пока нет
- Physiol Toric Calculator: With Abulafia-Koch Regression FormulaДокумент1 страницаPhysiol Toric Calculator: With Abulafia-Koch Regression FormuladeliОценок пока нет
- Ocular Trauma: H. Amiruddin, DR., Spm. Rsud Arifin Achmad PekanbaruДокумент22 страницыOcular Trauma: H. Amiruddin, DR., Spm. Rsud Arifin Achmad PekanbaruNovasiska Indriyani HutajuluОценок пока нет
- ICO Residency-Curriculum PDFДокумент219 страницICO Residency-Curriculum PDFJujunОценок пока нет
- Hyphema in GoatДокумент3 страницыHyphema in GoatMuhammad HasnainОценок пока нет
- CataractДокумент50 страницCataractApoorva Agrawal100% (3)
- Presbyopia Measurement TechniquesДокумент48 страницPresbyopia Measurement TechniquesAzmaОценок пока нет
- Swollen EyelidsДокумент4 страницыSwollen EyelidsNARENDRAОценок пока нет
- Eye Bank & Corneal GraftingДокумент48 страницEye Bank & Corneal Graftingshraddha chaudharyОценок пока нет
- CH 15Документ8 страницCH 15naguguОценок пока нет
- The Art of Medical Management in Glaucoma PDFДокумент29 страницThe Art of Medical Management in Glaucoma PDFCahyaОценок пока нет
- Morning Report MataДокумент14 страницMorning Report MataYoungky PutraОценок пока нет
- Cover Sarpus 1 GREДокумент7 страницCover Sarpus 1 GREGracia HarahapОценок пока нет
- Shanghai Child Eye Study Tracks Myopia ProgressionДокумент27 страницShanghai Child Eye Study Tracks Myopia ProgressionPutra TridiyogaОценок пока нет
- Dr. Stefan Sacu ResearchДокумент7 страницDr. Stefan Sacu Researchpeiras2011Оценок пока нет
- Ocutech CatalogДокумент14 страницOcutech CatalogHarpreet CheemaОценок пока нет
- Form Offline GifДокумент67 страницForm Offline Gifdpkppni dinkesОценок пока нет
- Retinal Occlusion As An Advanced Complication of Sickle Cell DiseaseДокумент7 страницRetinal Occlusion As An Advanced Complication of Sickle Cell DiseaseMuhammad Irfan FaizОценок пока нет
- Trust, Compassion and Loyalty Seeing ClearlyДокумент5 страницTrust, Compassion and Loyalty Seeing Clearlysvetlana_sdОценок пока нет
- ACCOMMODATIVE ESOTROPIA: CAUSES, TYPES & TREATMENTДокумент10 страницACCOMMODATIVE ESOTROPIA: CAUSES, TYPES & TREATMENTRishabh GuptaОценок пока нет
- Pediatric OptometryДокумент34 страницыPediatric OptometryUduma Kalu0% (1)
- Red Eye, Conjuctivitis, Vision LossДокумент213 страницRed Eye, Conjuctivitis, Vision LossShashi Royal TNОценок пока нет
- RD Symptoms Causes TreatmentsДокумент2 страницыRD Symptoms Causes Treatmentswieka mawieОценок пока нет
- Healthy Eyes and Ears Unit 16Документ4 страницыHealthy Eyes and Ears Unit 16CARMIN INES GOMEZ RAMIREZОценок пока нет
- Ocular Trauma - BantaДокумент211 страницOcular Trauma - BantaLuisa Fernanda Arboleda100% (1)
- Endothelial KeratoplastyДокумент17 страницEndothelial KeratoplastyZulva Fuadah AОценок пока нет
- Ocular AnatomyДокумент56 страницOcular AnatomyJulianto KeceОценок пока нет
- Lesson Plans - VisionДокумент5 страницLesson Plans - VisionZach SanfordОценок пока нет
- Introduction To Rabbit Eye ExperimentДокумент35 страницIntroduction To Rabbit Eye ExperimentSriram SunilОценок пока нет
- New ProposalДокумент29 страницNew ProposalBless CoОценок пока нет
- Health VisionДокумент24 страницыHealth VisionBhebe Castillo Delos ReyesОценок пока нет
- The Age of Magical Overthinking: Notes on Modern IrrationalityОт EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityРейтинг: 4 из 5 звезд4/5 (13)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsОт EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsРейтинг: 5 из 5 звезд5/5 (1)
- Why We Die: The New Science of Aging and the Quest for ImmortalityОт EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityРейтинг: 3.5 из 5 звезд3.5/5 (2)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsОт EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsРейтинг: 3.5 из 5 звезд3.5/5 (3)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionОт EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionРейтинг: 4 из 5 звезд4/5 (402)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeОт EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeОценок пока нет
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedОт EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedРейтинг: 5 из 5 звезд5/5 (78)
- Techniques Exercises And Tricks For Memory ImprovementОт EverandTechniques Exercises And Tricks For Memory ImprovementРейтинг: 4.5 из 5 звезд4.5/5 (40)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisОт EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisРейтинг: 4 из 5 звезд4/5 (1)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsОт EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsРейтинг: 4.5 из 5 звезд4.5/5 (169)
- The Obesity Code: Unlocking the Secrets of Weight LossОт EverandThe Obesity Code: Unlocking the Secrets of Weight LossРейтинг: 5 из 5 звезд5/5 (4)
- The Ultimate Guide To Memory Improvement TechniquesОт EverandThe Ultimate Guide To Memory Improvement TechniquesРейтинг: 5 из 5 звезд5/5 (34)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessОт EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessРейтинг: 4.5 из 5 звезд4.5/5 (327)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsОт EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsОценок пока нет
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingОт EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingРейтинг: 5 из 5 звезд5/5 (4)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeОт EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeРейтинг: 4.5 из 5 звезд4.5/5 (253)
- The Happiness Trap: How to Stop Struggling and Start LivingОт EverandThe Happiness Trap: How to Stop Struggling and Start LivingРейтинг: 4 из 5 звезд4/5 (1)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisОт EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisРейтинг: 4.5 из 5 звезд4.5/5 (41)
- The Stress-Proof Brain: Master Your Emotional Response to Stress Using Mindfulness and NeuroplasticityОт EverandThe Stress-Proof Brain: Master Your Emotional Response to Stress Using Mindfulness and NeuroplasticityРейтинг: 4.5 из 5 звезд4.5/5 (109)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingОт EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingРейтинг: 3.5 из 5 звезд3.5/5 (33)
- The Tennis Partner: A Doctor's Story of Friendship and LossОт EverandThe Tennis Partner: A Doctor's Story of Friendship and LossРейтинг: 4.5 из 5 звезд4.5/5 (4)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaОт EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisОт EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisРейтинг: 5 из 5 звезд5/5 (8)