Академический Документы
Профессиональный Документы
Культура Документы
The Grinding or Honing Method
Загружено:
Joseph Rosales Dela CruzИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
The Grinding or Honing Method
Загружено:
Joseph Rosales Dela CruzАвторское право:
Доступные форматы
THE GRINDING OR HONING METHOD I.
The Unstained Bone Materials: Fine toothed saw or jewelers saw Long bone (any) Small wide mouth bottle with cover Dissecting set Alcohol lamp Microscope slide Honing stone with rough & fine sides or sand paper, rough & fine Soap Cover glass Canada balsam Procedure: Clamp and hold the long bone firmly and with the use of the fine toothed saw, make the thin section of the lone bone. Slow sawing action is necessary to avoid chipping off of the outer and inner edges of the bone. It is often necessary and helpful if a gradual circular sectioning is done to the bone since a more or less even section can be made one at a time. Thinning to its final form can follow as soon as the cross sectioning is done. If the honing stone is used, the bone section should constantly be wet with water as it is being grinded. This is a long, slow and gradual process which if one become careless may result in rubbing off ones finger print and would lead the bleeding of the finger. To prevent this unpleasant experience, it is best recommended that the smooth surface of the bone be glued on a slide and rub gently against the honing stone or sand paper. Some workers prefer to cover the tip of their fingers with a piece of cloth or adhesive plaster while actual grinding is done. Examine the bone under the microscope every now and then to determine the desirable thickness. Be sure to wash the bone with soap and water before every examination under the microscope is done. Bone debris often times deceive in the beginning in knowing the correct thickness of the specimen. Immediately before reaching the correct thickness, rub the bone to the finer side of the honing stone or to the fine sand paper to make the surface even and smooth. Divide the bone to small pieces in such a way that each piece will have the outer and inner sides. The scalpel may be used. Wash thoroughly the specimen in soap and water. Shaking the specimen in a bottle of soap and water will easily remove the dirt. Rinse in water and dry on the air.
Groove Specimen String Green Papaya Fruit
Figure 6. Plant specimen tied between green papaya fruit for free hand sectioning
Test Tube holder Alkali Solution Specimen
Alcohol Lamp
Figure 7. Dissociation and maceration of spicules with the use of heat and alkali.
STAINING CHROMOSOMES USING ACETOCARMINE IN ONIO MITOSIS Objectives 1. Prepare a slide of stained chromosomes using the squash technique. 2. Identify the stages of mitosis from prepared stained slides of onion root tips seen under the compound microscope. 3. Identify the structures visible under the compound microscope of cells in various stages of cell division. 4. The solutions you need are (formulas to make most of these will follow below): a. Aceto-carmine stain b. Carnoys fixative c. 1N Hydrochloric acid d. canadabalsam
PROCEDURE Each human originates with the fertilization of an egg. At this stage of development the individual consists of a single cell about the size of the period at the end of this sentence. Division of this cell and many divisions of sequential cells over a period of several years may result in the formation of an adult human, consisting of about 1018 cells. Among these are cells of many kinds, each having a different structure, function, and life span. This process of mitosis, cell division, continues as long as the human or parts of his body are alive. In this investigation you will see in the various stages of mitosis. Staining Chromosomes Procedures: 1. With forceps, plane an onion root tip in the center of a clean slide. Using a scalpel or razor blade, cut off the terminal cm of the tip of the root. Dispose of the rest of the root. 2. Cover immediately with 3 or 4 drops of the 1N HCl. The acid is to act on the root tip for 5 minutes. During this period the slide is to be heated gently. This is done by placing the slide on the diffused light source for 5to 7 minutes. The onion root tip must be covered with the HCl solution during this warming period. 3. Blot off the acid without touching the root tip and cover the root tip with 2 or 3 drops of the acetocarmine solution. The stain is to set for about 5 minutes and add a cover glass. 4. Blot off the stain solution carefully. Add 2 drops of fresh stain solution carefully and add a cover glass. 5. With the slide flat on the table, place your thumb on the cover glass exactly above the specimen. Press straight down hard. 6. Blot the back of the slide and examine on low power. Look for embryonic cells. They appear square. When you find mitotic figures, switch to high power.
III. Capture the image or draw and label cells interphase, prophase, metaphase, anaphase, and telophase stages of mitosis from slides you prepared. STAGES OF MITOSIS By definition: Prophase begins when chromosomes are visible with the light microscope and continues until the nuclear membrane is no longer visible. begins when nuclear membrane is no longer visible. Chromosomes are in mass in the center of the cell. begins when the identical chromosomes start moving toward opposite ends of the cell begins with the invagination of cell membrane in animal tissue and with the first visible evidence of a forming cell plate in plant cells.
Metaphase
Anaphase
Telophase
Interphase chromosomes not visible with light microscope (not a stage of mitosis) 1. Describe the prominent cell morphology as seen through the compound microscope of animal tissue in each of the following phases: Interpahse Anaphase
Prophase
Telophase
Metaphase
2. Normal cell functions such as protein synthesis and respiration occur during which phase? 3. The duplication of DNA and chromatids occurs during which phase? 4. If a parent cell has 32 chromosomes, then after mitosis daughter cells each have how many chromosomes? 5. Contrast the telopahse in animal and plant cells.
MACERATION AND DISSOCIATION I. The animal Specimen: Spicules of sponges Materials and reagents: Sponge Microscope Cover glass Test Tube Dissecting needle Procedure: 1. Cut a piece of sponge with the use of scalpel or a pair scissors. 2. Transfer the sponge into the pyrex test tube with NaOH and boil over the alcohol flame un- the sponge is macerated. 3. Pour into the Petri dish with water and remove the debris. Change the water several times to remove the NaOH. See figure 7. 4. Decant the water and dry on the air or in the drier if available. 5. The spuicules with that of forceps and trans- to the microscope slide. 6. Put 2 to 3 drops of Canada balsam and lay over the cover glass. 7. Dry, clean and label. Questions: 1. What types of spicules in your slide? 2. What are their functions in the sponge? 3. How are the spicules formed in the sponge? Canada Balsam Alcohol lamp Test tube holder Petri dish NaOH, 5% Scalpel or scissors
12. Wash in five changes of water. 13. Carefully pick one shoot and put it on the microscope slide. Crush or moderate the shoot with use of dissecting needle and scalpel. 14. Melt Kaisers glycerine jelly on the spatula over the alcohol flame and pour 3 to 4 drops on the material. 15. Lay over the cover glass before Kaisers glycerine jelly solidify. 16. Melt paraffin on the spatula over the alcohol flame and seal the edges of the cover glass. 17. Dry, clean and label. Questions: 1. Identify, draw and label the structures in your prepared slide. What the functions of each structure? HONING METHOD I. The Animal Specimen: Long bone Materials and reagents: Fine toothed saw Fine and rough sand paper Honing stone Microscope slide Cover glass Procedure: 1. Hold or clamp a piece of long bone on a hard surface and with the use of fine toothed saw, cut thin sections from the outer to the inner sides of the bone. 2. Slowly rub the out surface of the bone occasionally wetting the surfaces with water. Even pressure of the finger is necessary to insure flat surface on the bone. 3. From time to time examine the bone under the microscope. When the lacunae and canaliculi become discernible, rub the bone surface against fine sand paper to remove the scratches on the surface. Fine sand papering should be done to both surface of the bone. 4. Trim the piece of bone about 5 mm. wide from the outer side to the inner side with scalpel. 5. Wash thoroughly with soap and water. Dry on the air and mount in Canada balsam. 6. Dry, clean and label. Questions: 1. 2. 3. 4. Why are the canaliculi dark or black in the unstained bone? Where is the bone cells located? How do the bone cells receive nourishment from the blood? How do bones grow? Microscope Scalpel Canada balsam Petri dish
WHOLEMOUNT METHOD I. The Animal Specimen: Head louse Materials and reagents: Head louse Cover glass Formalin, 10% Canada balsam Forceps Beaker, 50 ml. Microscope slide Medicine dropper Grades of alcohol 30%, 50%, 70%, 85%, 95%, 100% Procedure: 1. Kill and fix the head louse in 10% formalin overnight. Wash in 3 to 4 changes of water in the beaker. 2. Decant the water from the beaker and run-up for three minutes each in 30%, 50%, 70%, 85%, 95%, 100% alcohol. This is done by inclining the beaker to one side. With medicine dropper put several drops of 3% alcohol and this drained as dehydration time is completed. It is then replaced by the next higher grade of alcohol and so on. 100% alcohol shall be the last and should remain in the beaker. 3. Add xylene drop by as the beaker is agitated. Discard the mixture when the total volume is about xylene and alcohol. 4. Add pure xylene. Replace it if it turns cloudy. 5. Mount in Canada balsam. 6. Dry, clean and label. II. The Plant Specimen: Algae Hydrilla Materials and reagents: Navashins fluid Kaisers glycerine-gelatin Glycerine, pure & 10% Alcohol lamp Spatula Hydrilla Procedure: 1. Fix the desired size of Hydrilla in Navashins fluid in the beaker overnight. 2. Wash in running water. In the absence of running water, soak the Hydrilla in several changes of water. 3. Decant the water and add 10% glycerine for one hour. Replace the 10% glycerine with pure glycerine and leave it for another hour. Beaker, 50ml Microscope slide Cover glass filter paper or tissue paper Paraffin
4. Carefully pick the Hydrilla with forceps and lay over the microscope slide. Touch the Hydrill with a piece of filter paper to absorb as much glycerine as possible. 5. Scoop the Kaisers glycerine-gelatin with the spatula and heat to melt over the alcohol flame. 6. Put about 7 drops over the Hydrilla and lay over the cover glass before the gelatin solidify. 7. Melt paraffin chip on the spatula over the alcohol flame and seal the edges of the cover glass. 8. Clean, dry and label. Question: 1. Compose a procedure in staining Hydrilla before mounting on the microscope slide.
WHOLEMOUNT METHOD IN PLASTIC Specimens: Materials: Procedure: 1. Lay the dried starfish upside down in the paper cup or beaker. Pour premixed casting resin until the starfish is completely submerged. Put metal weight if the starfish float. It must remain submerged. The presence of rising bubbles to the surface is an indication the casting resin is impregnating the starfish. Cover the paper cup with tissue paper. Leave the specimen overnight. 2. Apply release mold compound with camels brush to the inner surfaces of the mold. Two or three coatings will make the mold leak proof. Dry on the air. 3. To a disposable tin can receptacle, pour 10 ml. premixed casting resin and add 5 to 7 drops of hardener. Mix thoroughly using the stirring rod but being careful not to stir in bubbles. Pour into the mold. Set aside for 2 to 4 hours or overnight until the resin has gelled and can support the weight of the starfish. This is the supporting layer of the starfish. Starfish, dried & small Glass or wood mold slightly bigger than starfish Casting resin, premixed Hardener Tissue paper Forceps Paper cups or tin receptacles Camels hair brush Medicine dropper Paraffin oven or drier Sand papers, water proof of 180, 250, 600, 800 grits Liquid polish or metal polish Cotton cloth or pelt polishing board Wood stirring rod Mold release compound Starfish or any dried animal and plant specimen
4. Using the forceps, remove the starfish from the premixed resin and lay over blotting paper. This to drain most of the resin. 5. Transfer the starfish into the mold up-side-down. Arrange the final position. In two hours the starfish will adhere to the supporting layer. 6. To another receptacle, pour 40 ml. of premixed casting resin. Add 20 to 28 drops of hardener. Thoroughly mix the hardener and the resin. Avoid stirring in bubbles. 7. Pour carefully into the mold. Be sure the surface of the resin is far above the starfish. Cover the mold with tissue paper to protect it from dust. Set aside overnight. 8. Transfer the mold into the paraffin oven or drier of 600C for 3 to 4 hours. At the end of this time switch off the oven and allow the mold to cool inside until room temperature. 9. Shake or tap the mold to remove the cast. It should easily separate from the mold due to the mold release compound. 10. Lay the Corse sand paper 180 grit on the flat surface and grid all the cast surfaces. Occasionally wet the sand paper with water. Use the next sandpaper of smaller grit and again grind all the cast surfaces. Wet also the sandpaper as you continue grinding. Continue changing to the next sandpaper until the finest grit. 11. Rub the cast to a piece of cloth saturated with liquid polish. The cast become transparent as it is rubbed against the liquid polish. The longer the cast is ribbed the more transparent the cast become. 12. Wash the cast with soap and water. Wipe it dry.
SPREADING METHOD Animal Specimen: Frog squamous epithelium
Materials and reagents: Petri dish Forceps Dissecting needle Epidermal cells Grades of alcohol, 30%, 50% 50%, 70%, 85%, 95%, 100% Xylene Canada balsam Microscope slide Cover glass Hematoxylin Mayers albumin NH4OH, 0.5% HCI, 1% Microscope
Procedure: 1. Stir the formalin in the frog preserving tank and collect the semitransparent peeled frog skin which float near the surface with the use of forceps. 2. Wash in Petri dish with water in several changes. 3. Spread thin film of Mayers albumin with clean finger on the microscope slide. 4. Hold the microscope slide in inclined position with left hand and bring it near to the epidermis. With the dissecting needle held by the right hand, drag or push the epidermis
to the surface of the slide. Scratch or spread the epidermis with the use of the dissecting needle. Set it aside for overnight. See figure 8. 5. Arrange in sequence from left to right the reagents in staining jars, water, hematoxylin, water, 30%, 50%, 70%, 85%, 95%, 100% alcohol and xylene. 6. Dip the slide in water and leave in hematoxylin for 30 minutes or until the nuclei have absorbed excess stain. 7. Distain by dipping the slide in HCl two times, quickly rinse in water and transfer without delay to NH4 OH. The epidermis should turn from pink to blue. Examine under the microscope. If the nuclei are still over stained, repeat the process of distaining until the desired color intensity is attained. 8. Leave the slide in two more minutes in water and run up for thirty seconds each in 30%, 50%, 70%, 85%, 95%, 100% alcohol. 9. Transfer to xylene until the epidermis is clear or transparent. Mount in balsam. 10. Dry, clean and label. Questions: 1. What other parts of your body can you find squamous epithelium? 2. Make an outline how squamous epithelium can be prepared by maceration and dissociation technique.
I. The Plant Specimen:
Onion root tip
Materials and reagents: Onion bulb Side mouthed bottle Farmers fluid HCl, 1N Filter paper Gum label Scalpel Spatula Microscope Procedure: 1. Lay the onion bulb with its base cleaned of dried roots over the wide mouthed bottle filled with water. Be certain the basal portion is immersed in water. 2. Set aside for 3 to 4 days until the roots appear to about one half inch long. 3. Cut off the root tips of 5 mm. long and immediately fix in Farmers Fluid in the beaker for one to two days. 4. Decant the Farmers Fluid and replace it with 1N HCl and set aside for 1 to 2 days or until the root tips are soft and fragile. 5. Decant the HCl. Tie the gauze or cheese cloth around the mouth of the beaker and wash in running water for 30 minutes or until the acid is completely removed. 6. Pick just one root tip with forceps and place it on the microscope slide. 7. With the scalpel squash or crush the root tips to separate the cells. 8. Put a drop of aceto-carmine over the cells. Focus under the microscope and observe the nuclei absorbing the stain. 9. Lay over the cover glass when the nuclei have sufficiently absorbed the stain. If necessary a drop of stain may be added to increase the intensity of stain. 10. Excess stain which flow beyond the edges of the cover glass can be removed by touching it with filter paper. 11. Melt paraffin chips in the spatula over the alcohol flame and seal the edges of the cover glass. 12. Clean, dry and label. Questions: 1. In what part of the root has the fastest rate of cell division? 2. What is the probable time of the day when many of the cells are undergoing mitosis? Conduct an experiment to support your answer. Outline below your plan of action. Beaker, 50ml. Microscope slide Cover glass Paraffin Alcohol lamp Camels hair brush Aceto-carmine Razor blade Piece of thread
II. The Plant Specimen:
Midrib, stem and root
Materials and reagents: Root, stem, midrib Scalpel or razor blade Beaker, 50 ml. Gauze or cheese cloth Tissue carrier Paper box Paper boat FAA Tissue holder Haupts adhesive Formalin, 3% Grades of alcohol, ethyl 30%, 50%, 70%, 85%, 95%, 100% Xylene
Xylene-alcohol mixture: 25 parts xylene & 75 parts of ethyl alcohol 50 parts xylene & 50 parts of ethyl alcohol 75 parts xylene & 25 parts of ethyl alcohol Xylene-paraffin mixture: 50 parts xylene & 50 parts soft paraffin 100 parts of soft paraffin 50 parts soft paraffin & 50 parts hard paraffin 100 parts of pure hard paraffin Procedure: 1. Cut the plant specimen into pieces of 5 mm and fix in formal acetic acid alcohol solution (FAA) in the beaker for at least 24 hours. 2. Decant the FAA and tie the gauze or cheese cloth around the mouth of the beaker. Wash in running water for 2 to 3 hours. See figure 10. 3. Transfer the specimen to the tissue carrier and dehydrate for 60 minutes each in gradually increasing grades of ethyl alcohol 30%, 50%, 70%, 85%, 95%, 100%. Bear in mind that alcohol is volatile and must be in covered bottles. Specimens must never be allowed to dry on air while being transferred from one grade of alcohol to another. 4. The specimens still in the tissue carrier are cleared gradually for 60 minutes in each of the increasing concentration of xylene: 25 parts xylene & 75 parts of 100% alcohol 50 parts xylene & 50 parts of 100% alcohol 75 parts xylene & 25 parts of 100% alcohol 100 parts xylene 5. Infiltrate the specimen with melted paraffin which have been placed in the paraffin oven of 600C to 650C. Leave the specimen for 60 minutes each in: 50 parts xylene & 50 parts soft paraffin 100 parts soft paraffin 50 parts soft paraffin & 50 parts hard paraffin 100 parts of hard paraffin
6. Moisten the lining of paper boat with glycerine and pour melted pure hard paraffin sufficient to cover the specimen when placed into it. 7. Without delay heat the forceps over the alcohol flame, pick a piece of specimen and immediately transfer to it designated location and position in the paper boat. This must be done to every specimen until all are in their respective designated location in the paper boat. Should the surface of the paraffin harden, heat the spatula over the alcohol flame and pass over the surface. The paraffin will melt and allow more time in the arrangement of the specimen. Bubbles which might have been formed may be removed by touching them with red hot dissecting needle. 8. Carefully lift with both hands the paper boat in horizontal position and float it on cold water. Gently blow your breathe over the surface of the paraffin to hasten hardening as you push down the paper boat with both hands. Sink the boat completely in water when the paraffin has solidified and can withstand water pressure. 9. Unfold and separate the paper boat from the solidified paraffin block. Leave the paraffin block in cool dry place for 24 hours to complete hardening. 10. With scalpel, divide the paraffin block into parts, each of which contains one specimen. These should at least be a margin on 10mm from all sides of the specimen. In blocks of square sides, opposite sides must be parallel to one another. 11. Heat the tissue holder which have been saturated with hard paraffin and pressed against the paraffin block. The tissue holder will firmly attach to the paraffin block as it hardens. 12. Clamp the tissue holder into the lamp holder of the rotary microtome. Section the paraffin block not more than 20 microns. 13. Arrange the tissue ribbon in the paper box from left to right and in rows from top to bottom so that the serial order may be comparable to the arrangement of the alphabet and the words on a printed page of the book. Tissue ribbon must be kept in dry cool place for storage. See figure 14. 14. Spread thin film of Haupts adhesive on the microscope slide the flood with 5 to 7 drops of water. Use medicine dropper. 15. Using the scalpel divide the paraffin ribbon into paraffin sections. Each paraffin section contains one tissue section. 16. Dip the tip of the scalpel in water and insert under the tissue section. The tissue section should adhere to the tip of the scalpel. 17. Lay flat the tissue section on the slide. The tissue section must float. If not, add water until it is freely floating. 18. Hold the slide with one hand and pass over the alcohol flame to warm the water only. The tissue section will expand and flatten as the water is warmed. 19. Decant the water by inclining the slide to one end. Arrange the tissue section according to its designated location on the slide. Keep inside the slide box overnight to dry or dry in the paraffin oven for thirty minutes. 20. Arrange the staining jars with their contents as in figure. 21. Warm the tissue slide over the alcohol flame and immerse in Xylol I to dissolve the paraffin. 22. Run down the tissue slide one minute each grade of alcohol from 100%, 95%, 85%, 70%, 50%, 35%. 23. Stain in 1% alcoholic safranin in 50% alcohol for four hours.
24. Wash the tissue slide in several changes of water until bleeding of stain from the tissue stop. 25. Run up the tissue slide from 50%, 70%, 85%, 95% alcohol for one minute each and 100% for three to five minutes. 26. Clear gradually for two minutes each in: 25 parts xylene & 75 parts of 100% ethyl alcohol 20 parts xylene & 50 parts of 100% ethyl alcohol 75 parts xylene & 25 parts of 100% ethyl alcohol 100 parts xylene 27. Mount in Canada balsam. 28. Dry, clean and label. Question: 1. From your prepared slide, draw and label the parts of the root, midrib and stem in cross section.
2. The Plant Specimen:
Onion root tip
Materials and reagents: Onion bulb Forceps Scalpel or razor blade Spatula Gauze or cheese cloth Dissecting needle Tissue carrier Glycerine Tissue holder Mayers albumin Paper boat Alcohol lamp Paper box Microscope slide Canada balsam Cover glass Small wide mouthed bottles for: 75 parts 100% alcohol & 25 parts xylene 50 parts 100% alcohol & 50 parts xylene 25 parts 100% alcohol & 75 parts xylene Small tin cans to be place in the paraffin oven: 75 parts xylene & 25 parts soft paraffin 50 parts xylene & 50 parts soft paraffin 25 parts xylene & 75 parts soft paraffin pure soft paraffin 75 parts soft paraffin & 25 parts hard paraffin 50 parts soft paraffin & 50 parts hard paraffin 25 parts soft paraffin & 75 parts hard paraffin pure hard paraffin Procedure: 1. Lay the onion bulb with its base cleaned of dried roots over the wide mouthed bottle filled with water. Be sure the basal portion is immersed in water. 2. Set it aside for 3 to 4 days until the roots are about one half inch long. 3. Cut off the tips 5 mm. long and immediately fix in Bouins fluid in the beaker for 1 to 2 days. 4. Decant the Bouins fluid and tie the gauze to cover the beakers mouth. Wash in running water until the yellow color is removed. 5. Carefully transfer the root tip to the tissue carrier and dehydrate for 30 to 45 minutes each in gradually increasing grade of alcohol 30%, 50%, 70%, 85%, 95%, 100%. 6. Clear the root tip for 45 to 60 minutes each in the gradually increasing concentrating of xylene: 75 parts 100% alcohol & 25 parts xylene 50 parts 100% alcohol & 50 parts xylene 25 parts 100% alcohol & 75 parts xylene pure xylene
7. Infiltrate liquid paraffin into the root tip. This is done in the paraffin oven of 600C to 650C for 30 to 45 minutes in each of the following mixture: 75 parts xylene & 25 parts soft paraffin 50 parts xylene & 50 parts soft paraffin 25 parts xylene & 75 parts soft paraffin pure soft paraffin 75 parts soft paraffin & 25 parts hard paraffin 50 parts soft paraffin & 50 parts hard paraffin 25 parts soft paraffin & 75 parts hard paraffin pure hard paraffin 8. Into the paper r boat which has been moistened with glycerine, pour melted pure hard paraffin. Carefully scoop the root tip with spatula and immediately transfer to the designated location and position in the paper boat. Air bubbles which might have been formed must be removed by touching them with red hot dissecting needle. 9. Hold and carry the paper boat in horizontal position and float on the old water. When the surface of the paraffin has solidified, push the paper boat downward and immerse underwater. Hold it under water for few minutes until the paraffin is firm and hard. 10. Unfold and separate the paper boat from the paraffin block. It may be kept in cool dry place for storage and complete hardening. 11. Trim the paraffin block with scalpel leaving about 3 to 4 mm. margin from the root tip. Opposite sides must be parallel to one another. 12. Heat the tissue holder over the alcohol flame and press it against the paraffin block. Melted hard paraffin may be added to the base of the paraffin block to insure its strong attachment to the tissue holder. 13. Clamp the tissue holder to the clamp holder of the rotary microtome. Section 8 to 10 microns. 14. Arrange the tissue ribbon in their serial order in the paper box, just as the order of words in a sentence. The first tissue section is at the left most and the last is in the right most end. If the tissue ribbon is too long, it may be divided into certain length and arranged in rows. The continuation of the right most section in one row is found in the left most section of the lower row. See figure 14. 15. To affix or adhere the tissue section on the microscope slide, spread thin film of Mayers albumin on the slide. Add several drops of water. Divide the tissue ribbon according to the length of the cover glass. Wet the tip of the scalpel with water. Pick the tissue sections and arrange them is serial order and in rows as they can be accommodated by the cover glass. Figure 15. 16. Warm evenly the microscope slide over the alcohol flame to spread the tissue sections. Drain the water by inclining the microscope slide to one end. Arrange the final position of the tissues with the use of the dissecting needle. Set aside in the slide box overnight to dry. 17. Arrange the staining jars with contents as in Figure 13. 18. Warm the tissue slide over the alcohol flame and immerse in xylol I to dissolve the paraffin. 19. Run down the tissue slide for 30 seconds in such grade of alcohol from 100%, 95%, 85%, 70%, 50%, 30%, water.
20. Mordant in 2% FeCl3, solution for 2 minutes. Dip in water 3 to 5 times and leave in Heiden-hains hemotoxylin for 12 to 24 hours. 21. Wash by dipping the tissue slide 5 to 7 times in water. Examine under the microscope. 22. If only if the chromosomes have absorbed excess stain, dip the tissue slide in 2% FeCl 3 solution to remove the excess stain. Examine under the microscope and observe the regression of stain in the chromosome. Continue the destaining proceed until the desired color intensity is reached and then was in water. 23. If, the chromosome has lost the stain during destaining process, re-stain by leaving the tissue slide again in Heidenhains hematoxylin. 24. Rinse the tissue slide in several changes of water to remove completely the FeCl 3 solution. 25. Run-up the tissue slide for 30 seconds in each grade of alcohol from 30%, 50%, 70%, 85%, 95%, 100%. Never let the tissue to dry on the air while it is being transferred from one grade of alcohol to another. 26. Clear the tissue slide in 2 changes of xylene. Mount in Canada balsam. 27. Dry, clean and label.
Вам также может понравиться
- Root Tip Mitosis LabДокумент2 страницыRoot Tip Mitosis LabJohn OsborneОценок пока нет
- Observing MitosisДокумент3 страницыObserving MitosisAnonymous tlSLEPRReОценок пока нет
- Staining Techniques Used in MicrobiologyДокумент21 страницаStaining Techniques Used in MicrobiologyBarry AllenОценок пока нет
- Negative, Simple and GramДокумент4 страницыNegative, Simple and GramMaddieОценок пока нет
- Cheek and Onion Peel Exp Xlass 9Документ9 страницCheek and Onion Peel Exp Xlass 9Rakshit YadavОценок пока нет
- Animal and Plant Cell Lab ActivityДокумент9 страницAnimal and Plant Cell Lab ActivityGexel CecilioОценок пока нет
- Experiment 1.1: Compound Light Microscope MaterialsДокумент9 страницExperiment 1.1: Compound Light Microscope MaterialsAlia MaisarahОценок пока нет
- Bio NotesДокумент71 страницаBio Notesrashmi_harryОценок пока нет
- Class 9 Science Lab Manual - Slide of Onion Peel and Cheek CellsДокумент6 страницClass 9 Science Lab Manual - Slide of Onion Peel and Cheek CellsPrerak0% (1)
- Practice 1 - Experiments To StudyДокумент5 страницPractice 1 - Experiments To Studyshreyavjha2020Оценок пока нет
- Lab Report - Garlic Root TipДокумент3 страницыLab Report - Garlic Root Tipgabriel8gavi8oОценок пока нет
- CP5 - Pupil Lab Report BookletДокумент7 страницCP5 - Pupil Lab Report Bookletalisonhan0210Оценок пока нет
- MitosisДокумент4 страницыMitosis2003rahulcreatorОценок пока нет
- Anatomy and PhysiologyДокумент138 страницAnatomy and PhysiologyMikee GatlabayanОценок пока нет
- Observing Mitosis in Garlic Roots (40Документ14 страницObserving Mitosis in Garlic Roots (40Muhammad Marsaid Bin KardiОценок пока нет
- FALLSEM2022-23 BBIT204P LO VL2022230102119 Reference Material I 11-11-2022 Karyotyping-10Документ6 страницFALLSEM2022-23 BBIT204P LO VL2022230102119 Reference Material I 11-11-2022 Karyotyping-10Dhanush KumarОценок пока нет
- Material and Procedure L6 - AliffДокумент2 страницыMaterial and Procedure L6 - Aliffalipokada99Оценок пока нет
- Genetics Study of Cell DivisionДокумент83 страницыGenetics Study of Cell DivisionSurabhiОценок пока нет
- Gram Stain & Microscopy TechniquesДокумент17 страницGram Stain & Microscopy TechniquesKaycee Gretz LorescaОценок пока нет
- Lab Report 1Документ7 страницLab Report 1lordyayaОценок пока нет
- Staining NotesДокумент5 страницStaining NotesSwetha RameshОценок пока нет
- 9 Practical - 1Документ5 страниц9 Practical - 1anubh50Оценок пока нет
- Ex. 1 Wet Mount PreparationДокумент2 страницыEx. 1 Wet Mount PreparationAphril Joy LoberianoОценок пока нет
- Core Practical Experiments Unit 2: Root Tip SquashДокумент9 страницCore Practical Experiments Unit 2: Root Tip SquashHsia Ang100% (1)
- Cell Structure: Procedure For Viewing Prepared SlidesДокумент4 страницыCell Structure: Procedure For Viewing Prepared Slidesaditi_joshee419Оценок пока нет
- STD 12 Biology - Practical Record WorkДокумент33 страницыSTD 12 Biology - Practical Record WorkGopika Ramesh100% (1)
- Objective::: Om Pattnayak: XiiДокумент12 страницObjective::: Om Pattnayak: XiiOm PattnayakОценок пока нет
- Anatomy Protocols 2Документ7 страницAnatomy Protocols 2Monica FigueroaОценок пока нет
- Biology Investigatory Project 121126091042Документ12 страницBiology Investigatory Project 121126091042ajitsingh220867% (3)
- Experiment BioДокумент29 страницExperiment BioQin Jamil100% (1)
- LessonsДокумент16 страницLessonsThư TrầnОценок пока нет
- BIO EXP 1 and 2Документ7 страницBIO EXP 1 and 2Bala Murugan.VОценок пока нет
- Lab 2: Streaking For Isolation, Microscopy, and Simple Staining BIO 2440: Principles of MicrobiologyДокумент4 страницыLab 2: Streaking For Isolation, Microscopy, and Simple Staining BIO 2440: Principles of MicrobiologyDivergent64Оценок пока нет
- Laboratory 4: - Meiosis, MitosisДокумент4 страницыLaboratory 4: - Meiosis, MitosisSabyashashi SaikiaОценок пока нет
- Experiment 2 BIO 111Документ5 страницExperiment 2 BIO 111melvinchilambeОценок пока нет
- Biology 3rd Lab Report (Animal Cell)Документ4 страницыBiology 3rd Lab Report (Animal Cell)Johanna Haludilu67% (3)
- Bio83 - LabAct No. 2 - Bacterial Smear and StainingДокумент5 страницBio83 - LabAct No. 2 - Bacterial Smear and StainingMaej OragaОценок пока нет
- Class 9 Experiment 4 Onion Cell, Cheek CellДокумент3 страницыClass 9 Experiment 4 Onion Cell, Cheek CellARSHLEEN KAUR GHAIОценок пока нет
- Core Practical 5Документ8 страницCore Practical 5Sana NainaОценок пока нет
- LabManual 2-3Документ5 страницLabManual 2-3Christian Jayvon LalunaОценок пока нет
- Osmosis in Onion Cell LabДокумент2 страницыOsmosis in Onion Cell Lab7ghkw9rsshОценок пока нет
- UntitledДокумент5 страницUntitledLaila EmeraldОценок пока нет
- Micro - Power PointДокумент48 страницMicro - Power PointGalana BiratuОценок пока нет
- SAPS - Root Tip Mitosis For A-Level Set Practicals - Student NotesДокумент4 страницыSAPS - Root Tip Mitosis For A-Level Set Practicals - Student NotesRashida HanifОценок пока нет
- Techniques in Microbiology I PDFДокумент16 страницTechniques in Microbiology I PDFMohd Izwan67% (3)
- Observing Mitosis in Onion Root TipsДокумент2 страницыObserving Mitosis in Onion Root TipsNurul Amni ZulkefleyОценок пока нет
- Onion Root Cell MitosisДокумент3 страницыOnion Root Cell MitosisSherin SunnyОценок пока нет
- Bio411 Lab Report 2Документ25 страницBio411 Lab Report 2Nur Aqillah100% (1)
- Stain SimpleДокумент3 страницыStain Simplelux0008Оценок пока нет
- Biology Experiment #2Документ2 страницыBiology Experiment #2Grace JosephОценок пока нет
- PraticalДокумент5 страницPraticalsanjayshrivas092Оценок пока нет
- Lab Report 1.3 BioДокумент4 страницыLab Report 1.3 BioAmran NazriОценок пока нет
- Bio 122 Lab ReportДокумент25 страницBio 122 Lab ReportWilbert WanОценок пока нет
- Week 3 Lab Arado, Patrick James M.Документ2 страницыWeek 3 Lab Arado, Patrick James M.Jeffry AradoОценок пока нет
- 3 Mic125Документ8 страниц3 Mic125nadiazkiОценок пока нет
- Biology Practical 3 AS LevelДокумент4 страницыBiology Practical 3 AS LevelMr TОценок пока нет
- Activity 1 - Preparing Plant and Animal Cell SlidesДокумент4 страницыActivity 1 - Preparing Plant and Animal Cell Slidesjilmorata2Оценок пока нет
- Experiment To Observe Temporary Mount of A Leaf Peel To Show StomataДокумент3 страницыExperiment To Observe Temporary Mount of A Leaf Peel To Show StomataEliseo Pamandanan0% (1)
- Laboratory - Viewing Animal and Plant Cell Under The MicroscopeДокумент3 страницыLaboratory - Viewing Animal and Plant Cell Under The MicroscopeCortez Faye Nicole L.Оценок пока нет
- A Survey Questionnaire On The SocioДокумент2 страницыA Survey Questionnaire On The SocioJoseph Rosales Dela Cruz100% (2)
- Aknowledgement ReceiptДокумент2 страницыAknowledgement ReceiptJoseph Rosales Dela CruzОценок пока нет
- Special Power or AttorneyДокумент1 страницаSpecial Power or AttorneyJoseph Rosales Dela CruzОценок пока нет
- ATTENDANCEДокумент1 страницаATTENDANCEJoseph Rosales Dela CruzОценок пока нет
- LECTURE TOPICS FOR INQUIRIESДокумент3 страницыLECTURE TOPICS FOR INQUIRIESJoseph Rosales Dela CruzОценок пока нет
- PNPACAT Medical Certificate Form PDFДокумент1 страницаPNPACAT Medical Certificate Form PDFShizuko Shasimie Kazue48% (31)
- Affidavit of Loss: NEWAGAC, GATTARAN, CAGAYAN After Having Been Duly Sworn To in AccordanceДокумент1 страницаAffidavit of Loss: NEWAGAC, GATTARAN, CAGAYAN After Having Been Duly Sworn To in AccordanceJoseph Rosales Dela CruzОценок пока нет
- Special GuavaДокумент2 страницыSpecial GuavaJoseph Rosales Dela CruzОценок пока нет
- Item Analysis Dressmaking/Tailoring Grade 9: Submitted By: Leticia A. Miguel Master Teacher IДокумент6 страницItem Analysis Dressmaking/Tailoring Grade 9: Submitted By: Leticia A. Miguel Master Teacher IJoseph Rosales Dela CruzОценок пока нет
- Affidavit of Non-ReceiptДокумент1 страницаAffidavit of Non-ReceiptJoseph Rosales Dela CruzОценок пока нет
- Man's Cultural Evolution from Paleolithic to Post-Industrial SocietiesДокумент1 страницаMan's Cultural Evolution from Paleolithic to Post-Industrial SocietiesJoseph Rosales Dela CruzОценок пока нет
- 5000 TOEFL Words PDFДокумент36 страниц5000 TOEFL Words PDFPrudhveeraj Chegu100% (2)
- 26 6 PDFДокумент1 страница26 6 PDFJoseph Rosales Dela CruzОценок пока нет
- Provincial Human RecourceДокумент1 страницаProvincial Human RecourceJoseph Rosales Dela CruzОценок пока нет
- My Life TestimonyДокумент6 страницMy Life TestimonyJoseph Rosales Dela Cruz100% (1)
- Ipcrf ArnelynДокумент2 страницыIpcrf ArnelynJoseph Rosales Dela Cruz100% (1)
- Muscular System DiseasesДокумент5 страницMuscular System DiseasesJoseph Rosales Dela CruzОценок пока нет
- Aquatic PlantsДокумент6 страницAquatic PlantsJoseph Rosales Dela CruzОценок пока нет
- Mr. Jackston C. PentecostosДокумент1 страницаMr. Jackston C. PentecostosJoseph Rosales Dela CruzОценок пока нет
- Presiden 1Документ4 страницыPresiden 1Joseph Rosales Dela CruzОценок пока нет
- Republic of The PhilippinesДокумент1 страницаRepublic of The PhilippinesJoseph Rosales Dela CruzОценок пока нет
- Kidney StructureДокумент9 страницKidney StructureJoseph Rosales Dela CruzОценок пока нет
- Self-development plan templateДокумент3 страницыSelf-development plan templateJoseph Rosales Dela CruzОценок пока нет
- CoverДокумент17 страницCoverThabz ChubyОценок пока нет
- Term Paper in Personal ManagementДокумент5 страницTerm Paper in Personal ManagementJoseph Rosales Dela CruzОценок пока нет
- Certification: April Joy S. BorcelasДокумент1 страницаCertification: April Joy S. BorcelasJoseph Rosales Dela CruzОценок пока нет
- Aquatic Plants: Nelumbo Nucifera, Also Known As Indian Lotus, Sacred Lotus, Bean of India, or Simply Lotus, IsДокумент6 страницAquatic Plants: Nelumbo Nucifera, Also Known As Indian Lotus, Sacred Lotus, Bean of India, or Simply Lotus, IsJoseph Rosales Dela CruzОценок пока нет
- CrisДокумент2 страницыCrisJoseph Rosales Dela CruzОценок пока нет
- CERTIFICATIO6Документ1 страницаCERTIFICATIO6Joseph Rosales Dela CruzОценок пока нет
- San Jacinto Construction Service and GenДокумент6 страницSan Jacinto Construction Service and GenJoseph Rosales Dela CruzОценок пока нет
- Final NPRD, 2021Документ28 страницFinal NPRD, 2021Maruthi RoshanОценок пока нет
- 2nd ICBB ProceedingsДокумент117 страниц2nd ICBB ProceedingsLenie Angeles Quiatchon-BaezaОценок пока нет
- Microbiology NotesДокумент3 страницыMicrobiology NotesAthena Huynh100% (1)
- Differential Diagnosis and Comorbidity Distinguishing Autism From Other Mental Health Issues NeuropsychiatryДокумент11 страницDifferential Diagnosis and Comorbidity Distinguishing Autism From Other Mental Health Issues NeuropsychiatryJohn Bowen BrownОценок пока нет
- HepatosplenomegalyДокумент52 страницыHepatosplenomegalySundar NatarajanОценок пока нет
- Pharmacology MCQ PebcДокумент36 страницPharmacology MCQ Pebcsnowden1100% (6)
- Therapeutic Relationship NotesДокумент11 страницTherapeutic Relationship NotesAlexandra StanОценок пока нет
- Lecture Notes For Mental Health Nursing Psych NursingДокумент84 страницыLecture Notes For Mental Health Nursing Psych NursingAnn Michelle Tarrobago100% (2)
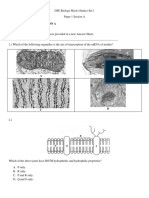
- DSE Biology Mock (Sunny) Set 1 Paper 1 Section AДокумент18 страницDSE Biology Mock (Sunny) Set 1 Paper 1 Section AwslОценок пока нет
- Basal Ganglia Physiological AspectsДокумент54 страницыBasal Ganglia Physiological AspectsPhysiology by Dr Raghuveer100% (1)
- Surgery Papillary Thyroid CAДокумент15 страницSurgery Papillary Thyroid CAMelissa LabadorОценок пока нет
- Introduction of MicrobiologyДокумент9 страницIntroduction of MicrobiologyRahul PalsОценок пока нет
- Prenatal Diagnosis: Asheber Gaym M.D. January 2009Документ7 страницPrenatal Diagnosis: Asheber Gaym M.D. January 2009Israel WoseneОценок пока нет
- HCC Surveillance in An Era of BiomarkersДокумент48 страницHCC Surveillance in An Era of BiomarkersRobert G. Gish, MDОценок пока нет
- Plant Pathogens Principles of Plant PathologyДокумент376 страницPlant Pathogens Principles of Plant PathologyChirag GuptaОценок пока нет
- Journal Reading - TLR9 Expression Correlates with Cytokine Levels in SLE PatientsДокумент48 страницJournal Reading - TLR9 Expression Correlates with Cytokine Levels in SLE PatientsEdwin DarmawanОценок пока нет
- ICSE Class 10 Biology Sample Papers 2 2021Документ8 страницICSE Class 10 Biology Sample Papers 2 2021Reet KhimavatОценок пока нет
- Clinical Success of Deproteinization in Hypocalcified Amelogenesis ImperfectaДокумент7 страницClinical Success of Deproteinization in Hypocalcified Amelogenesis ImperfectaMelisa GuerraОценок пока нет
- Psychology Chapter 5 NotesДокумент6 страницPsychology Chapter 5 NotesMoses SuhОценок пока нет
- BM7122 Module Handbook 2021-22 v1Документ10 страницBM7122 Module Handbook 2021-22 v1MasoomaIjazОценок пока нет
- NonClassical MHC Class I PDFДокумент12 страницNonClassical MHC Class I PDFRobMarvinОценок пока нет
- Dendrimers: Synthesis, Applications, and Properties: Nanoreview Open AccessДокумент10 страницDendrimers: Synthesis, Applications, and Properties: Nanoreview Open AccessIonut StirbescuОценок пока нет
- Quantitative Analysis of The Substrate Specificity of Human Rhinovirus 3C Protease and Exploration of Its Substrate Recognition MechanismsДокумент14 страницQuantitative Analysis of The Substrate Specificity of Human Rhinovirus 3C Protease and Exploration of Its Substrate Recognition MechanismsLuís MiguelОценок пока нет
- Summary of Powerpoint Presentation On ObesityДокумент2 страницыSummary of Powerpoint Presentation On ObesityYolieОценок пока нет
- PRM - Ma5 BookletДокумент25 страницPRM - Ma5 BookletVan LabasanoОценок пока нет
- Funda - NursingДокумент29 страницFunda - NursingLloyd Rafael EstabilloОценок пока нет
- JC-1 Dye For Mitochondrial Membrane Potential - Thermo Fisher ScientificДокумент4 страницыJC-1 Dye For Mitochondrial Membrane Potential - Thermo Fisher Scientificankitsaneja1Оценок пока нет
- My Power Subliminals DescriptionsДокумент13 страницMy Power Subliminals DescriptionsAayansh RihaanОценок пока нет
- PDF Lecture 9 Benign Soft Tissue TumorsДокумент131 страницаPDF Lecture 9 Benign Soft Tissue TumorsMuhammad Rizqi100% (1)
- The Reproductive SystemДокумент119 страницThe Reproductive SystemAshis karmakar100% (10)