Академический Документы
Профессиональный Документы
Культура Документы
ANTI-FLAGÒ M2 Magnetic Beads
Загружено:
Roberto Riquelme NeiraИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
ANTI-FLAGÒ M2 Magnetic Beads
Загружено:
Roberto Riquelme NeiraАвторское право:
Доступные форматы
ANTI-FLAG M2 Magnetic Beads Catalog Number M8823 Storage Temperature 20 C
TECHNICAL BULLETIN
Product Description ANTI-FLAG M2 Magnetic Beads are composed of the murine derived, ANTI-FLAG M2 monoclonal antibody attached to superparamagnetic iron impregnated, 4% agarose beads with an average diameter of 50 m. The M2 antibody binds to fusion proteins containing the FLAG peptide sequence.1 Additionally, the M2 antibody recognizes the FLAG octapeptide sequence (N-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys-C) at the N-terminus, Met-N-terminus, or C-terminus locations of a fusion protein in mammalian and bacterial extracts. The ANTI-FLAG M2 Magnetic Beads are useful for detection and capture of fusion proteins containing a FLAG peptide sequence by commonly used immunoprecipitation procedures. The magnetic properties allow for very rapid separation of the beads from a suspension, significantly accelerating manipulations, such as repetitive washings or processing of multiple samples performed in multiwell plates. This leads to faster experimentation, better reproducibility, and more accurate quantitation of the proteins of interest. Binding capacity: 0.6 mg of FLAG fusion protein per 1 ml of packed magnetic beads. Specificity: 90% specificity towards FLAG fusion proteins from mammalian and bacterial cell extracts. Reagent The ANTI-FLAG M2 Magnetic Bead resin is supplied as a 50% suspension in 50% glycerol with 10 mM sodium phosphate, 150 mM sodium chloride, pH 7.4, and 0.02% (w/v) sodium azide (PBA/A). Precautions and Disclaimer This product is for R&D use only, not for drug, household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices. Equipment Required But Not Provided Magnetic Separators for: Microcentrifuge tubes (Catalog Number M1167) Tissue culture flasks (Catalog Number M1292) Centrifuge tubes (Catalog Number M1542) Magnet for 96-well tissue culture plates (Catalog Number SHM05) Magnetic plate for standard sized well plates, T-25 through T-75 tissue culture flasks, and up to 5 cm dishes (Catalog Number SHM04) Do not use a magnetic stirring system. This will destroy the resin beads. Storage/Stability The ANTI-FLAG M2 Magnetic Beads ship on wet ice and storage at 20 C is recommended. The product is supplied in a 50% glycerol solution with preservative and is stable for 2 years at 20 C. After use, the resin should be cleaned and stored in 50% glycerol with TBS or PBS buffer containing preservative to protect the product. Freezing the magnetic beads in the absence of 50% glycerol will irreversibly damage the bead structure. Procedures Note: It is recommended the entire technical bulletin be read before use, especially the Reagent Compatibility Table at the end of this bulletin. There are many different procedures for performing small-scale affinity capture experiments. The following procedures are written for a single sample. For batch-wise purification, 100 l of the resin bead suspension per reaction (50 l of packed gel) is recommended. For use in a 96-well plate format, 10 l of packed gel is recommended per well. The amount of resin bead can be varied depending on the amount of target protein in the sample and the type of magnetic separator utilized.
Part I. Sample Preparation 1. Adjust the pH of the protein extract to between pH 78. It is also useful to adjust the salt concentration with sodium or potassium chloride to 0.15 M in the protein extract to prevent a large amount of nonspecific protein binding to the resin. 2. The FLAG fusion protein extract must be clarified to remove any insoluble material. A large amount of insoluble material may require centrifugation (10,00020,000 g for 15 minutes) for removal. The protein extract should also be filtered through a 0.45 or 0.22 m filter to remove any remaining cell debris and particulates that may interfere with protein binding. Part II. Binding Procedures For purification of FLAG fusion proteins, the resin can be used in either a batch or 96-well plate format. For larger volumes of lysate, the batch format is recommended to quickly capture the target protein from a large volume of extract. If a smaller sample is being purified, the FLAG fusion protein can be immunoprecipitated. A. Automation Format for ANTI-FLAG M2 Magnetic Beads - Please see our website (www.sigma.com/ automation) for a purification procedure for FLAG fusion proteins using the KingFisher automated platform. B. Batch Format for Absorption of FLAG Fusion Proteins with ANTI-FLAG M2 Magnetic Beads - This procedure provides a quick and efficient way to purify FLAG fusion proteins from a dilute solution. Table 1. Binding Capacity of Beads Packed Gel Volume (l) 20 50 100 200 Binding Capacity (g) 12 30 60 120
Do not allow the resin to remain in TBS (50 mM Tris HCl, 150 mM NaCl, pH 7.4) buffer for extended periods of time (>24 hours) unless an antimicrobial agent (e.g., 0.02% sodium azide) is added to the buffer. 1. Thoroughly resuspend the resin by gentle inversion. Make sure the bottle of ANTI-FLAG M2 Magnetic Beads is a uniform suspension. Remove an appropriate volume for use (see Table 1). 2. Transfer resin to an appropriate sized tube. Place tube in the appropriate magnetic separator to collect the beads. Remove and discard storage buffer. 3. Equilibrate beads by resuspending with 5 packed gel volumes of TBS (50 mM Tris HCl, 150 mM NaCl, pH 7.4). Mix thoroughly. 4. Place tube in the appropriate magnetic separator to collect the beads. Remove and discard TBS buffer. 5. Repeat steps 3 and 4 once. Allow a small amount of buffer to remain on the top of the beads. 6. Incubate the protein extract (see Sample Preparation) with the equilibrated beads (step 5) for 1 hour at room temperature with gentle mixing to capture the FLAG fusion proteins. Mixing should be done on either a rotating device or a platform shaker. Do not use a magnetic stirring system. This will destroy the resin beads. 7. Once the binding step is complete, collect the magnetic beads by placing the tube in the appropriate magnetic separator and remove the supernatant. 8. Wash the resin beads with TBS buffer to remove all of the nonspecifically bound proteins. Washing should be done with 20 packed gel volumes of TBS buffer, performed in three sequential bead washing. Note: The washing process can be monitored by measuring the absorbance of the supernatant at 280 nm. Continue washing the resin until the absorbance difference between the wash solution aspirated from the beads and the wash solution (TBS) blank is <0.05. 9. The FLAG proteins can be eluted from the magnetic beads either by low pH or by competition with the FLAG peptide. a. Elution of FLAG fusion proteins under acidic conditions with glycine - Elute the bound FLAG fusion protein from the magnetic beads with 10 packed gel volumes of 0.1 M glycine HCl, pH 3.0, collecting 1 packed gel volume of eluate in a vial containing 1525 l of 1 M Tris, pH 8.0. Do not leave eluate in the glycine HCl solution for longer than 20 minutes. Reequilibrate the resin to neutral pH as soon as possible after elution. Or
The ANTI-FLAG M2 Magnetic Beads are stored in 50% glycerol with buffer. The glycerol must be removed just prior to use and the resin equilibrated with buffer (steps 15). The equilibration can be done at room temperature or at 28 C. Remove only the volume of resin that is necessary for the purification (see Table 1).
b. Elution of FLAG fusion proteins by competition with the FLAG Peptide Elute the bound FLAG fusion protein by competitive elution with five packed gel volumes of a solution containing the FLAG peptide (100 g/ml, Catalog Number F3290) in TBS buffer. 10. Cleaning the Magnetic Beads - It is recommended the beads be cleaned immediately after use by washing with three packed gel volumes of 0.1 M glycine HCI, pH 3.0. The beads should be immediately re-equilibrated in TBS buffer until the effluent is at neutral pH. 11. Storing the Magnetic Beads - After cleaning the beads, collect the magnetic beads by placing the tube in the appropriate magnetic separator and remove the buffer. The beads may be stored as a 50% suspension in 50% glycerol with TBS or PBS buffer containing 0.02% sodium azide. Store the beads at 28 C or 20 C without draining. C. Format for Immunoprecipitation of FLAG Fusion Proteins using ANTI-FLAG M2 Magnetic Beads - This procedure is recommended for the purification of small amounts of FLAG fusion proteins. Note: For antigens and protein:protein complexes requiring a special lysis buffer composed of a different percentage of detergent, it is recommended to pretest the resin before use. The ANTI-FLAG M2 resin bead is resistant to the many detergents at the following concentrations: 5.0% TWEEN 20, 5.0% TRITON X-100, 0.1% IGEPAL CA-630, 0.1% CHAPS, and 0.2% digitonin. It can also be used with 1.0 M NaCl or 1.0 M urea. See the Reagent Compatibility Table for use with additional chemicals. The following procedure is an example of a single immunoprecipitation reaction. For multiple immunoprecipitation reactions, calculate the volume of reagents needed according to the number of samples to be processed. For immunoprecipitation reactions, it is recommended to use 40 l of the 50% bead suspension per reaction (20 l of packed gel volume). Smaller amounts of resin (10 l of packed gel volume, which binds >1 g FLAG fusion protein) can be used. Note: Two control reactions are recommended for the procedure. The first control is immunoprecipitation with FLAG-BAP fusion protein (positive control) and the second is a reagent blank with no protein (negative control).
1. Thoroughly resuspend the resin by gentle inversion. Make sure the bottle of ANTI-FLAG M2 Magnetic Beads is a uniform suspension. Remove an appropriate volume for use (see Table 1). To lessen damage to beads, it is recommended to cut the end of the pipette tip. 2. Place tube in the appropriate magnetic separator to collect the beads. Aspirate and discard storage buffer. 3. Wash the packed gel twice with 10 packed gel volumes of TBS (50 mM Tris HCl, 150 mM NaCl, pH 7.4) buffer. Be sure most of the wash buffer is removed and no resin is discarded. Note: For multiple immunoprecipitation samples, wash the total volume of resin needed for all samples together. After washing, resuspend the resin in TBS buffer and divide the resin according to the number of samples tested. Place tube in the appropriate magnetic separator to collect the beads. Remove and discard TBS buffer. 4. Add 2001,000 l of cell lysate to the washed resin beads. If necessary, bring the final volume to 1 ml by adding lysis buffer (50 mM Tris HCl, pH 7.4, with 150 mM NaCl, 1 mM EDTA, and 1% TRITON X-100). The volume of cell lysate to be used depends on the expression level of FLAG fusion protein in the transfected cells. For the positive control, add 1 ml of TBS buffer and 4 l of 50 ng/l FLAG-BAP fusion protein (200 ng) to the washed resin beads. For the negative control, add 1 ml of lysis buffer only with no protein. The amount of FLAG-BAP fusion protein to be precipitated depends on the detection method. 200 ng of protein is sufficient for an activity assay or for an immunoblot analysis. For SDS-PAGE analysis with Coomassie blue or silver staining detection, use 1 g of FLAG-BAP fusion protein. 5. Agitate or shake (a roller shaker is recommended) all samples and controls gently for 2 hours. In order to increase the binding efficiency, the binding step may be extended overnight. 6. Place tubes in the appropriate magnetic separator to collect the beads and remove the supernatant with a narrow-end pipette tip. 7. Wash the resin three times with a total of 20 packed gel volumes of TBS (50 mM Tris HCl, 150 mM NaCl, pH 7.4). Make sure all the supernatant is removed by using a Hamilton syringe or equivalent device.
8. Elution of the FLAG fusion proteins - Three elution methods are recommended according to protein characteristics or further usage: Protein elution under native conditions by competition with 3X FLAG peptide. The elution efficiency is very high using this method. Elution under acidic conditions with 0.1 M glycine HCl, pH 3.0. This is a fast and efficient elution method. Neutralization of the eluted protein with wash buffer may help preserve its activity. Elution with sample buffer for gel electrophoresis and immunoblotting. Elution with 3X FLAG peptide a. Prepare 3X FLAG elution solution. Dissolve 3X FLAG peptide (Catalog Number F4799) in 0.5 M Tris HCl, pH 7.5, with 1 M NaCl at a concentration of 25 g/l. Dilute 5-fold with water to prepare a 3X FLAG stock solution containing 5 g/l of 3X FLAG peptide. For elution, add 3 l of 5 g/l 3X FLAG peptide stock solution to 100 l of TBS buffer (150 ng/l final concentration). b. Add 5 packed gel volumes of 3X FLAG elution solution to each sample and control resin. c. Incubate the samples and controls with gently shaking or on a rotator for 30 minutes at 28 C. d. Place tube in the appropriate magnetic separator to collect the beads. Transfer the supernatants to fresh tubes using a Hamilton syringe or equivalent device. Be careful not to transfer any resin. e. Repeat steps a-d, pooling eluates in the same tube. f. For immediate use, store the combined eluates at 28 C. Store at 20 C for long term storage. g. For cleaning and storage of used resin, see Batch Format Procedure, steps 10 and 11. Elution with 0.1 M Glycine HCl, pH 3.0 - The procedure should be performed at room temperature. Do not leave the resin in this buffer more than 20 minutes. a. Add 5 packed gel volumes of 0.1 M glycine HCl buffer, pH 3.5, to each sample and control resin. b. Incubate the samples and controls with gentle shaking or on a rotator for 5 minutes at room temperature.
c.
Place tube in the appropriate magnetic separator to collect the beads. Transfer the supernatants to fresh tubes containing 10 l of 0.5 M Tris HCl, pH 7.4, with 1.5 M NaCl, using a Hamilton syringe or equivalent device. Be careful not to transfer any resin. d. Repeat steps a-d, pooling eluates in same tube. e. For immediate use, store the combined eluates at 28 C. Store at 20 C for long term storage. f. For cleaning and storage of used resin, see Batch Format Procedure, steps 10 and 11. Elution with SDS-PAGE Sample Buffer - The procedure should be performed at room temperature. Sample buffer should be at room temperature before use. In order to minimize the denaturation and elution of the M2 antibody, no reducing agents, e.g., 2-mercaptoethanol or DTT, should be included in the sample buffer. The addition of reducing agents will result in the dissociation of the heavy and light chains of the immobilized M2 antibody (25 and 50 kDa bands). If reducing conditions are absolutely necessary, a reducing agent may be added. The final concentration of 2-mercaptoethanol or DTT in the 1 sample buffer (62.5 mM Tris HCl, pH 6.8, 2% SDS, 10% (v/v) glycerol, and 0.002% bromphenol blue) should be 5% or 50 mM, respectively. Note: Elution of the bound FLAG fusion protein as a SDS-PAGE sample results in damage to the ANTI-FLAG M2 Magnetic Beads and they cannot be used again. The SDS in the sample buffer will denature the M2 antibody and boiling will damage the bead structure. a. Add 20 l of 2 sample buffer (125 mM Tris HCl, pH 6.8, 4% SDS, 20% (v/v) glycerol, and 0.004% bromphenol blue) to each sample and control. b. Boil the sample and control tubes for 3 minutes. c. Place tubes in the appropriate magnetic separator to collect the beads. Transfer the supernatants to fresh tubes with a Hamilton syringe or a narrow-end Pasteur pipette. The samples and controls are ready for loading on SDS-PAGE and immunoblotting using ANTI-FLAG or specific antibodies against the fusion protein.
References 1. Brizzard, B.L., et al., Immunoaffinity purification of FLAG epitope-tagged bacterial alkaline phosphatase using a novel monoclonal antibody and peptide elution. BioTechniques, 16, 730-735 (1994). 2. Safarik, I., and Safarikova, M., Magnetic Techniques for the Isolation and Purification of Proteins and Peptides. BioMagnetic Research and Technology, 2, (2004). ANTI-FLAG and FLAG are registered trademarks of Sigma-Aldrich Biotechnology LP and Sigma-Aldrich Co. Reagent Compatibility Table Reagent Chaotropic agents (e.g., guanidine HCl or urea) Reducing agents (such as 2-mercaptoethanol, DTT, or DTE) TWEEN 20, 5% or less TRITON X-100, 5% or less IGEPAL CA-630, 0.1% or less CHAPS, 0.1% or less Digitonin, 0.2% or less Sodium chloride, 1.0 M or less Sodium dodecyl sulfate (SDS) Effects Denatures the immobilized M2 antibody
FLAG-BAP is a trademark of Sigma-Aldrich Biotechnology LP and Sigma-Aldrich Co. TWEEN is a registered trademark of Croda International PLC. Triton is a registered trademark of Union Carbide Corp. IGEPAL is a registered trademark of General Dyestuff Corp. Coomassie is a registered trademark of Imperial Chemical Industries Ltd. Hamilton is a registered trademark of Hamilton Co. KingFisher is a registered trademark of Thermo Fisher Scientific, Inc. JD,MAM 06/09-1
Reduces the disulfide bridges holding the M2 antibody chains together Reduces non-specific protein binding to the resin bead Reduces non-specific protein binding to the resin bead Reduces non-specific protein binding to the resin bead Reduces non-specific protein binding to the resin bead Reduces non-specific protein binding to the resin bead Reduces non-specific protein binding to the resin bead by reducing ionic interactions Denatures the immobilized M2 antibody
Comments Do not use any reagent that contains these types of components since it will denature the M2 antibody on the resin and destroy its ability to bind the FLAG fusion proteins. Low concentrations of urea (1 M or less) can be used. Do not use any reagent that contains these types of components since it will reduce the disulfide linkages in the M2 antibody on the resin and destroy its ability to bind the FLAG fusion proteins. May be used up to recommended concentration of 5%, but do not exceed. May be used up to recommended concentration of 5%, but do not exceed. May be used up to recommended concentration of 0.1%, but do not exceed. May be used up to recommended concentration of 0.1%, but do not exceed. May be used up to recommended concentration of 0.2%, but do not exceed. May be used up to recommended concentration of 1.0 M, but do not exceed. Do not use any reagent that contains this detergent in the loading and washing buffers since it will denature the M2 antibody on the resin bead and destroy its ability to bind the FLAG fusion proteins. SDS in the sample buffer is useful for the removal of proteins for immunoprecipitation, but the resin bead cannot be reused Do not leave the column in glycine HCl for longer than 20 minutes. Longer incubation times will begin to denature the M2 antibody Do not use any reagent that contains this detergent since it will inhibit the M2 antibody from binding to FLAG fusion proteins.
0.1 M glycine HCl, pH 3.5 Deoxycholate
Elutes FLAG protein from the resin bead Interferes with M2 binding to FLAG fusion proteins
Troubleshooting Guide Problem No signal is observed. Possible Cause FLAG fusion protein is not present in the sample. Solution Make sure the protein of interest contains the FLAG sequence by immunoblot or dot blot analyses. Prepare fresh lysates. Avoid using frozen lysates. Use appropriate protease inhibitors in the lysate or increase their concentrations to prevent degradation of FLAG fusion protein Reduce the number of washes. Avoid adding high concentrations of NaCl to the mixture. Use solutions that contain less or no detergent. Increase the incubation times with the affinity resin (from several hours to overnight). Lysates containing high concentrations of dithiothreitol (DTT), 2-mercaptoethanol, or other reducing agents may destroy antibody function, and must be avoided. Excessive detergent concentrations may interfere with the antibody-antigen interaction. Detergent levels in buffers may be reduced by dilution. If Western blotting detection is used: Check primary and secondary antibodies using proper controls to confirm binding and reactivity. Verify the transfer was adequate by staining the membrane with Ponceau S. Use fresh detection substrate or try a different detection system Pre-clear lysate with Mouse IgG-Agarose (Catalog Number A0919) to remove nonspecific binding proteins. After suspending beads for the final wash, transfer entire sample to a clean microcentrifuge tube before centrifugation Increase the number of washes. Increase duration of the washes, incubating each wash for at least 15 minutes. Increase the salt and/or detergent concentrations in the wash solutions. Centrifuge at lower speed to avoid nonspecific trapping of denatured proteins from the lysate during the initial centrifugation of the affinity resin complexes.
Washes are too stringent.
Incubation times are inadequate. Interfering substance is present in sample.
Detection system is inadequate.
Background is too high.
Proteins bind nonspecifically to the ANTI-FLAG monoclonal antibody, the resin beads, or the microcentrifuge tubes Washes are insufficient.
Sigma brand products are sold through Sigma-Aldrich, Inc. Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side of the invoice or packing slip.
Вам также может понравиться
- L00695 ManualДокумент4 страницыL00695 Manualandrea LuzardoОценок пока нет
- Western Blot (ABCAM)Документ5 страницWestern Blot (ABCAM)강지영Оценок пока нет
- Flag ResinДокумент8 страницFlag Resinhlee15Оценок пока нет
- ANTI-FLAG M2 Affinity Gel (A2220) - Technical BulletinДокумент8 страницANTI-FLAG M2 Affinity Gel (A2220) - Technical BulletinSigma-Aldrich100% (4)
- Sigma MALDI Calibration KitДокумент4 страницыSigma MALDI Calibration KitRamona Neka TamoОценок пока нет
- General Western Blot ProtocolДокумент7 страницGeneral Western Blot ProtocolMOHAMMAD NAZMUL ISLAMОценок пока нет
- Protein ProtocolsДокумент5 страницProtein ProtocolsFernanda Furlan Goncalves DiasОценок пока нет
- Isolation and Analysis of Urinary Glycosaminoglycans: ReagentsДокумент5 страницIsolation and Analysis of Urinary Glycosaminoglycans: ReagentsSeo Eun KimОценок пока нет
- Lsab KitДокумент9 страницLsab KitnutjabberОценок пока нет
- Profiling of Methyltransferases and Other S-Adenosyl - Homocysteine-Binding Proteins by Capture Compound Mass Spectrometry (CCMS)Документ7 страницProfiling of Methyltransferases and Other S-Adenosyl - Homocysteine-Binding Proteins by Capture Compound Mass Spectrometry (CCMS)Anh TranОценок пока нет
- PROTOCOLДокумент4 страницыPROTOCOLfama18Оценок пока нет
- Zymography of Metalloproteinases: UNIT 21.15Документ12 страницZymography of Metalloproteinases: UNIT 21.15Dr-Gnaneshwar GoudОценок пока нет
- C18 Spin Column Usage MethodДокумент8 страницC18 Spin Column Usage Methodphd.data19Оценок пока нет
- 1551f Veratox For Fumonisin 8830 8831 KitinsertДокумент12 страниц1551f Veratox For Fumonisin 8830 8831 KitinsertMartin BoninfanteОценок пока нет
- Immunostaining: Immunostaining - Paraffin and Frozen SectionsДокумент4 страницыImmunostaining: Immunostaining - Paraffin and Frozen SectionsIvana MedigovicОценок пока нет
- Nature in Gel Digestion Protocol 02 07 13Документ5 страницNature in Gel Digestion Protocol 02 07 13Dave ZОценок пока нет
- Sucrose Gradient Separation ProtocolДокумент16 страницSucrose Gradient Separation ProtocolabatabrahamОценок пока нет
- Myoglobin ELISA: For The Quantitative Determination of Myoglobin in Human SerumДокумент8 страницMyoglobin ELISA: For The Quantitative Determination of Myoglobin in Human SerumTanveerОценок пока нет
- Rat PDGF (Platelet-Derived Growth Factor) ELISA Kit: Instruction ManualДокумент9 страницRat PDGF (Platelet-Derived Growth Factor) ELISA Kit: Instruction ManualSigit Harya HutamaОценок пока нет
- Elisa MethodДокумент7 страницElisa MethodNandia SeptiyoriniОценок пока нет
- Human Testican 1 ELISA Kit (TIC1) Catalog Number RK02387: Manufactured byДокумент15 страницHuman Testican 1 ELISA Kit (TIC1) Catalog Number RK02387: Manufactured bycostaОценок пока нет
- Monoclonal ANTI-FLAG M2, Clone M2 (F3165) - Data SheetДокумент4 страницыMonoclonal ANTI-FLAG M2, Clone M2 (F3165) - Data SheetSigma-Aldrich100% (1)
- BIOLS 315: Biochemistry Lab. No. 3Документ7 страницBIOLS 315: Biochemistry Lab. No. 3Noor JanahiОценок пока нет
- Immunoprecipitation Protocol (IP)Документ6 страницImmunoprecipitation Protocol (IP)halfangle100% (1)
- Mouse IL-8 (Interleukin 8) ELISA Kit: Instruction ManualДокумент9 страницMouse IL-8 (Interleukin 8) ELISA Kit: Instruction ManualAak An NasherОценок пока нет
- IFU CHLG0070 Chlamydia Trachomatis IgG ELISA RUO 210923Документ8 страницIFU CHLG0070 Chlamydia Trachomatis IgG ELISA RUO 210923Jacob MadibaОценок пока нет
- Book Chapter Haider, Reid, SharpДокумент17 страницBook Chapter Haider, Reid, SharpmlОценок пока нет
- Insulin ELISA: Instructions For UseДокумент9 страницInsulin ELISA: Instructions For UseJosue Rojas ArayaОценок пока нет
- Insert Bioelisa SYPHILIS 3.0Документ8 страницInsert Bioelisa SYPHILIS 3.0Ika Afriliya WijayaОценок пока нет
- Exp 7-SDS-PAGEДокумент18 страницExp 7-SDS-PAGERadwan M SaadehОценок пока нет
- Metanephrine Plasma ELISA: Instructions For UseДокумент17 страницMetanephrine Plasma ELISA: Instructions For UseYousra ZeidanОценок пока нет
- Human DSP (Dentin Sialoprotein) ELISA KitДокумент9 страницHuman DSP (Dentin Sialoprotein) ELISA Kitmsk adiwiryaОценок пока нет
- MTT AssayДокумент2 страницыMTT AssayBatlia GonzalezОценок пока нет
- Glutathione Sepharose 4B: GE HealthcareДокумент26 страницGlutathione Sepharose 4B: GE HealthcareMadstcОценок пока нет
- 786-932 ProtocolДокумент12 страниц786-932 ProtocolShital BishtОценок пока нет
- Contaminating Microorganisms in Products With ProbioticsДокумент8 страницContaminating Microorganisms in Products With ProbioticsJose Alfredo DiazОценок пока нет
- GEHEALTH VivaspinДокумент16 страницGEHEALTH VivaspinAlzeniraОценок пока нет
- MDA Kits For Rat For Researcher OnlyДокумент14 страницMDA Kits For Rat For Researcher OnlyPritta TaradipaОценок пока нет
- VeratoxДокумент12 страницVeratoxLiliaMirandaОценок пока нет
- 43 Hahhu E01Документ8 страниц43 Hahhu E01Cao Thi Le ChiОценок пока нет
- SDS-PAGE: Laemmli System: SolutionsДокумент3 страницыSDS-PAGE: Laemmli System: SolutionsPaulita AracenaОценок пока нет
- tmpB9D2 TMPДокумент6 страницtmpB9D2 TMPFrontiersОценок пока нет
- IFU TOXM0460 Toxoplasma Gondii IgM - Capture ELISA RUO 210923 eДокумент8 страницIFU TOXM0460 Toxoplasma Gondii IgM - Capture ELISA RUO 210923 eTan Hung LuuОценок пока нет
- Sandwich Immunoassay KitДокумент8 страницSandwich Immunoassay KitDuyen KyОценок пока нет
- Protein Electrophoresis KitДокумент4 страницыProtein Electrophoresis KitrdctripletestОценок пока нет
- Principle of SDSДокумент3 страницыPrinciple of SDSZaid al GifariОценок пока нет
- Delfino CastelanДокумент9 страницDelfino CastelanJoseBuendiaОценок пока нет
- CSB E13415hДокумент14 страницCSB E13415hhuripОценок пока нет
- BN-PAGE For AnalysisДокумент9 страницBN-PAGE For AnalysisAG Khan100% (1)
- In Gel Tryptic DigestionДокумент6 страницIn Gel Tryptic DigestionNeha KumarОценок пока нет
- MTT Proliferation Assay ProtocolsdaДокумент2 страницыMTT Proliferation Assay ProtocolsdadwinugrohojuandaОценок пока нет
- BCA ProteinДокумент5 страницBCA ProteinDorelia SimonaОценок пока нет
- Total Dietary Fiber (Codex Alimentarius Definition) : ObjectiveДокумент12 страницTotal Dietary Fiber (Codex Alimentarius Definition) : ObjectiveMuhammad Farhan HidayatОценок пока нет
- Cusabio IgG SarsДокумент10 страницCusabio IgG SarsТатьяна ИсаеваОценок пока нет
- Method 3520C Continuous Liquid-Liquid ExtractionДокумент8 страницMethod 3520C Continuous Liquid-Liquid ExtractionridermateОценок пока нет
- Plant Protein Extraction Protocol For SDS PageДокумент7 страницPlant Protein Extraction Protocol For SDS PageAbhinay BatchuОценок пока нет
- Normetanephrine Plasma ELISA: Instructions For UseДокумент17 страницNormetanephrine Plasma ELISA: Instructions For UseYousra ZeidanОценок пока нет
- Analytical Characterization of BiotherapeuticsОт EverandAnalytical Characterization of BiotherapeuticsJennie R. LillОценок пока нет
- Experimental approaches to Biopharmaceutics and PharmacokineticsОт EverandExperimental approaches to Biopharmaceutics and PharmacokineticsОценок пока нет
- Journal of Cereal Science - v49 - Iss1Документ166 страницJournal of Cereal Science - v49 - Iss1Osama AbdulkareemОценок пока нет
- York County Court Schedule For Feb. 17Документ25 страницYork County Court Schedule For Feb. 17York Daily Record/Sunday NewsОценок пока нет
- Chapter 5 ProblemsДокумент3 страницыChapter 5 Problemswalt richards0% (1)
- Osho Talk On ReligionДокумент15 страницOsho Talk On ReligionOshoОценок пока нет
- NucleoSpin Tissue XS-ManualДокумент29 страницNucleoSpin Tissue XS-ManualL'bel Esika Cyzone ProductosОценок пока нет
- Course Coordinator & Lecturer Qihui Jiang (Vivi Kasim), PHD: Academic BackgroundsДокумент11 страницCourse Coordinator & Lecturer Qihui Jiang (Vivi Kasim), PHD: Academic BackgroundsRefsya Azanti PutriОценок пока нет
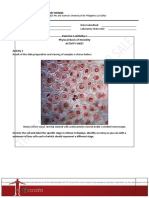
- Exercise 1-Activity 1 Physical Basis of Heredity: Name: Date Submitted: Lab Section: Laboratory InstructorДокумент8 страницExercise 1-Activity 1 Physical Basis of Heredity: Name: Date Submitted: Lab Section: Laboratory InstructorJansen AbadОценок пока нет
- BS-411 - 15 PDFДокумент1 страницаBS-411 - 15 PDFyoniОценок пока нет
- BIOLOGY Reviewer 1Документ17 страницBIOLOGY Reviewer 1Jellanne CañezОценок пока нет
- Astm A53Документ22 страницыAstm A53Juberthlan0% (2)
- Bioinformatics and Functional Genomics, Second Edition. by Jonathan PevsnerДокумент9 страницBioinformatics and Functional Genomics, Second Edition. by Jonathan PevsnerGabbsОценок пока нет
- Art:10.1007/s11240 017 1179 6Документ12 страницArt:10.1007/s11240 017 1179 6Wulan NursyiamОценок пока нет
- Human Genome ProjectДокумент28 страницHuman Genome Projectمحمد بلال سرورОценок пока нет
- Para, Gene DisДокумент11 страницPara, Gene DisMudit MisraОценок пока нет
- Multiple Choice Questions On Molecular BiologyДокумент6 страницMultiple Choice Questions On Molecular BiologyronojoysenguptaОценок пока нет
- IC407 Biomedical InstrumentationДокумент2 страницыIC407 Biomedical InstrumentationVaishnav PsОценок пока нет
- Quiz On Cell CycleДокумент9 страницQuiz On Cell CycleParnava Pratihar V BINOY 64Оценок пока нет
- Spur Beck 2004sДокумент18 страницSpur Beck 2004snvp16Оценок пока нет
- Biotechnology 2nd Edition Clark Test BankДокумент25 страницBiotechnology 2nd Edition Clark Test BankCameronAllenmtwei100% (58)
- Intro To DownstreamprocessingДокумент24 страницыIntro To DownstreamprocessingmluluОценок пока нет
- Summary of Infant Formula Test Results - Dr. SeidlerДокумент4 страницыSummary of Infant Formula Test Results - Dr. SeidlerStatesman Journal100% (1)
- Biochemistry Chapter 1Документ115 страницBiochemistry Chapter 1habteОценок пока нет
- About Us - Sigma-AldrichДокумент1 страницаAbout Us - Sigma-AldrichAndrew JohnsonОценок пока нет
- CV RemyДокумент6 страницCV RemyremythomasОценок пока нет
- Biotechnology Concept Map TestДокумент5 страницBiotechnology Concept Map TestWenny Pintalitna Tarigan SilangitОценок пока нет
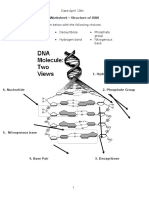
- Biology 12 Unit 5 Dna Worksheet - Dna Strucuture 1Документ2 страницыBiology 12 Unit 5 Dna Worksheet - Dna Strucuture 1api-338578874100% (1)
- Mep and Civil TenderДокумент471 страницаMep and Civil TenderJayadevDamodaran100% (1)
- Gfp-Model Proteína Verde Fluorescente Ag2014Документ2 страницыGfp-Model Proteína Verde Fluorescente Ag2014Christian HuertaОценок пока нет
- Ethanol Production by Wine FermentationДокумент6 страницEthanol Production by Wine FermentationSabyasachi DasguptaОценок пока нет
- CHI Catalog March2018 August2018Документ490 страницCHI Catalog March2018 August2018Glauce L TrevisanОценок пока нет