Академический Документы
Профессиональный Документы
Культура Документы
Lesson Plan
Загружено:
Nurul FadhilahИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Lesson Plan
Загружено:
Nurul FadhilahАвторское право:
Доступные форматы
LESSON PLAN FORM 1 Theme: Matter in Nature Learning Area: The Variety of Resources on the Earth Learning Objectives:
-To understand elements, compounds and mixtures Learning Outcome: By the end of the lesson, students will be able to:-state what elements, compounds and mixtures are (CoRT2 Organise; Recognise, Analyse and Compare) -give examples of elements, compounds, and mixtures, -state the differences between elements, compounds and mixtures, Activities: INDUCTION SET 1. Teacher distributes handout to students. By this time, teacher has placed all the materials needed on a few stations. Refer Task 1. 2. Teacher asks students to go to the stations to observe the materials using CoRT2Organise. 3. Teacher asks students to use CoRT1 CAF to classify the items on the station into elements, compounds, and mixtures by completing the task as required. 4. Students are given 5-10 minutes to complete this activity. 5. Then, teacher displays the correct answers. (refer attachment Task 1) LESSON DEVELOPMENT 6. Teacher asks: How do you classify those items? 7. Teacher explains about element using CoRT2 Consolidate. Do you know what elements are? (An element is a substance which cannot be broken down into two or more simpler substances by chemical methods. 8. Teacher asks students to use CoRT1 Rules to give more examples of elements. Can anyone give examples of elements? (sulphur, iron, aluminium, charcoal, gold, silver) 9. Teacher tells students that a compound is a substance which consists of 2 or more elements chemically combined together. Can anyone give any examples? (salt, marble, sand, wood, sugar, rice, carbon dioxide, glass) 10. A mixture consists of 2 or more substances which are not joined together chemically. Can anyone give some examples? (diluted acid, syrup, petrol, air, coffee, and crude oil) 11. Teacher asks students to plan using CoRT1 Planning to carry out activity 4.1 (refer text book). 12. Teacher asks a representative from each group to go in front and write their observations on the board. 13. Teacher discusses with students now they know that there are 2 methods to separate the components of a mixture. 14. Students are asked to complete a table (refer Task 2) using CoRT4 Combination and CoRT2 Organise. 15. Then, teacher discusses the correct observations students should get. 16. Briefly, teacher explains about elements and compounds using PowerPoint presentation. 17. Teacher asks students to compare between compound and mixture.
Language Focus: characteristics, classify, compound, element, dissolving, evaporation, metals, nonmetals Strategy: Cooperative Learning, self study Learning Styles: discussion, lecture, Project, simulation Values: Confident & independent, Objective, Critical Thinking, dare to try Reflection:
Notes: i) Homework: Then, teacher asks students to complete handout module chapter 4 given to them earlier (use CoRT2 Recognise)
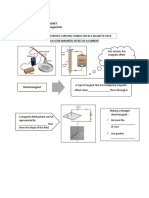
Task 1
CoRT1 CAF and CoRT2 Recognise
Sulphur powder
Crude oil
Iron Filings
Petrol
Salt
Plastic spoon
Sand
coffee
Aluminium coil
Syrup
Charcoal
Carbon dioxide
Marble Wood
Air Rice Silver ring
Iron filings & sulphur powder after heating
Dilute alkali
CoRT4 Combination and CoRT2 Organise Observation
Gold ring
Iron filings & sulphur powder
Sugar
Task 2
Name Colour Can the components be separated by magnet? Note any energy released or absorbed during the preparation? Examine the substance with a hand lens Add a little of each substance into a test tube containing carbon disulphide Add a little of each substance into a test tube containing dilute hydrochloric acid
Mixture of
Iron sulphide compound
Вам также может понравиться
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- EssayДокумент4 страницыEssayNurul FadhilahОценок пока нет
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- RPH F2 Potential and Kinetic EnergyДокумент2 страницыRPH F2 Potential and Kinetic EnergyNurul FadhilahОценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Answer SchemeДокумент5 страницAnswer SchemeNurul FadhilahОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- 46.2-Electromagnetic Effects-Cie Igcse Physics Ext-Theory-QpДокумент13 страниц46.2-Electromagnetic Effects-Cie Igcse Physics Ext-Theory-QpNurul FadhilahОценок пока нет
- Theme 2: Electric and Electromagnet Learning Area: 4.0 ElectromagnetismДокумент23 страницыTheme 2: Electric and Electromagnet Learning Area: 4.0 ElectromagnetismNurul FadhilahОценок пока нет
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Nuclear Physics 2Документ5 страницNuclear Physics 2Nurul FadhilahОценок пока нет
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- SOW Physics F3-F5 (2020-2022)Документ51 страницаSOW Physics F3-F5 (2020-2022)Nurul FadhilahОценок пока нет
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- NUCLEAR Tugasan f5Документ7 страницNUCLEAR Tugasan f5Nurul FadhilahОценок пока нет
- QuizДокумент5 страницQuizNurul FadhilahОценок пока нет
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- ElectricityДокумент200 страницElectricityNurul FadhilahОценок пока нет
- F1-C8 PHY LightДокумент78 страницF1-C8 PHY LightNurul FadhilahОценок пока нет
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Quiz: Forces in EquiibriumДокумент1 страницаQuiz: Forces in EquiibriumNurul FadhilahОценок пока нет
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Sumnative Test Sound Waves, Light, EnergyДокумент7 страницSumnative Test Sound Waves, Light, EnergyNurul FadhilahОценок пока нет
- 2016 Percubaan SPM Kedah - Biologi Kertas 2 PDFДокумент23 страницы2016 Percubaan SPM Kedah - Biologi Kertas 2 PDFNurul FadhilahОценок пока нет
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- 1.4 Sense of TasteДокумент3 страницы1.4 Sense of TasteNurul FadhilahОценок пока нет
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Unit IДокумент17 страницUnit IGoogle passwordОценок пока нет
- Class V Kamrup Metro 2018Документ25 страницClass V Kamrup Metro 2018JОценок пока нет
- Kenya EducationДокумент6 страницKenya Educationapi-265887966Оценок пока нет
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- July 22,2019Документ2 страницыJuly 22,2019Eldie OcarizaОценок пока нет
- Pretest 1Документ4 страницыPretest 1Jo Traven AzueloОценок пока нет
- Personal Philosophy of NursingДокумент5 страницPersonal Philosophy of Nursingapi-339774380Оценок пока нет
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The School Board Over Utah Job Corps - Google SearchДокумент1 страницаThe School Board Over Utah Job Corps - Google SearchLeroy ThompsonОценок пока нет
- Kurikulum Ib En-IndoДокумент9 страницKurikulum Ib En-IndoTK AL HIKMAH PRATAMAОценок пока нет
- Click HereДокумент0 страницClick HereD M Shawkot HossainОценок пока нет
- State Common Entrance Test Cell, Government of MaharashtraДокумент2 страницыState Common Entrance Test Cell, Government of Maharashtrathanecyber1Оценок пока нет
- KH Ilp CompletedДокумент4 страницыKH Ilp Completedapi-556629034Оценок пока нет
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Accomplishment Report Jan. 2023Документ2 страницыAccomplishment Report Jan. 2023MARIA ELSA CASTANEDAОценок пока нет
- Assignment - MBA-302 Operations ManagementДокумент3 страницыAssignment - MBA-302 Operations ManagementKirtiChoudharyОценок пока нет
- Health Seeking Behaviour and Healthcare Services in Rajasthan, India A Tribal Community's PerspectiveДокумент25 страницHealth Seeking Behaviour and Healthcare Services in Rajasthan, India A Tribal Community's PerspectiveThe IIHMR UniversityОценок пока нет
- AbubakarДокумент5 страницAbubakarJoshua MirandillaОценок пока нет
- Reservation System in IndiaДокумент50 страницReservation System in IndiaVivek BhardwajОценок пока нет
- How To Write A: Walking Bass LineДокумент3 страницыHow To Write A: Walking Bass LineShreyans Jain100% (2)
- L11 單字、會話Документ30 страницL11 單字、會話Cindy TsaiОценок пока нет
- Silver QSPДокумент422 страницыSilver QSPplandry78100% (1)
- Learning Lab User Guide 06242022 v1Документ7 страницLearning Lab User Guide 06242022 v1Rae PittmanОценок пока нет
- Awareness and Perception On Wildlife and ConservationДокумент9 страницAwareness and Perception On Wildlife and ConservationDenison CordovaОценок пока нет
- Module 2 JMДокумент11 страницModule 2 JMJordan Abosama MamalumpongОценок пока нет
- Bridging Between LanguagesДокумент14 страницBridging Between Languagestrisha abad80% (5)
- Surya International School Business Plan Final PDFДокумент36 страницSurya International School Business Plan Final PDFBahati Richardson80% (5)
- WPS Dongle2 User Manual enДокумент38 страницWPS Dongle2 User Manual ennias144Оценок пока нет
- Laura Rodríguez Rojas. Cause Effect EssayДокумент3 страницыLaura Rodríguez Rojas. Cause Effect EssayLaura RodriguezОценок пока нет
- Trends Grade 12 Monthly TestДокумент2 страницыTrends Grade 12 Monthly TestMichelle Labajo MaunesОценок пока нет
- Impact of Covid-19 On Employment Opportunities For Fresh Graduates in Hospitality &tourism IndustryДокумент8 страницImpact of Covid-19 On Employment Opportunities For Fresh Graduates in Hospitality &tourism IndustryInternational Journal of Application or Innovation in Engineering & ManagementОценок пока нет
- Rizal EssayДокумент1 страницаRizal EssayDonella BondocОценок пока нет
- Analisis - Komparasi - Sistem - Pendidikan - Indonesia - Dan Jurnal PDFДокумент20 страницAnalisis - Komparasi - Sistem - Pendidikan - Indonesia - Dan Jurnal PDFJihat AbdillahОценок пока нет