Академический Документы
Профессиональный Документы
Культура Документы
Acetylcholinesterase
Загружено:
Fajr MuzammilИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Acetylcholinesterase
Загружено:
Fajr MuzammilАвторское право:
Доступные форматы
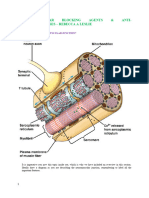
Acetylcholinesterase: A Representative Inactivation Enzyme
This is the side view of the human brain, facing left. The lights are showing you some of the places where neurons use acetylcholine as their neurotransmitter. We now zoom in on one of these neurons which uses acetylcholine. The cell body, located in the septal nucleus of the hypothalamus, sends its axon to the hippocampus. At the end of the axon is the terminal bouton. After the arrival of an action potential, vesicles containing acetylcholine fuse with the presynaptic membrane and release acetylcholine into the synaptic cleft.
Acetylcholine (ACh), the first neurotransmitter ever to be identified, is a smallmolecule excitatory neurotransmitter with a wide variety of known functions. Within the central nervous system, cholinergic cells (neurons that use ACh as a neurotransmitter) are found in several different locations of the brain, including the striatal complex, the basal forebrain, the diencephalon, pontomesencephalic cell groups, and the medulla. Depending on which area of the brain it is found in, ACh may be involved in any one of several different functions. Some of these functions include the conduction of pain, the regulation of neuroendocrine function, the regulation of REM sleep cycles, and the process of learning and memory formation. In the sympathetic and parasympathetic nervous systems and at all neuromuscular junctions, ACh is used to signal muscle movement. Whether it is within the brain, or at the neuromuscular junction, when ACh is released into the synaptic cleft, it transiently binds with a ligand-gated receptor molecule on the post-synaptic neuron or muscle cell. This binding causes the receptor molecule to open up, allowing for an exchange of ions which subsequently results in a depolarization of the post-synaptic neuron. If this depolarization is strong enough, an action potential is stimulated. After depolarization, the neurotransmitter must be swiftly removed from the receptor molecule and inactivated in order to prevent constant stimulation of the post-synaptic membrane and an excessive firing of action potentials. ACh is one of several neurotransmitters whose action at the synaptic cleft is terminated by the hydrolyizing action of an enzyme. Once ACh has been recognized and the postsynaptic membrane has been depolarized, ACh quickly dissociates from the receptor and binds to acetylcholinesterase (AChE), an enzyme named for its ability to break the ester bond that holds acetate and choline together. Removal of ACh from the recognition site causes the membrane-spanning pore of the receptor to close. The electric potential of the membrane then normalizes and the action potential ceases to fire. The breakdown of ACh by AChE inactivates the neurotransmitter and prevents it from reassociating with the receptor molecule.
///////////
IVA2. Location, Structure and Function of Acetylcholinesterase Presynaptic vesicles release acetylcholine into the synaptic cleft where it binds to its receptor. Right next to the receptor is acetylcholinesterase, the enzyme which breaks up acetylcholine into acetate and choline. AChE is a glycoprotein that exists is several forms. For images of acetylcholinesterase's structure displayed in ribbon form, go tohttp://jsdnt.claremont.edu/biochem98/acetylcholinesterase/AChEcontents.html. As a result of the variety in chemical structure, some forms of AChE are hydrophobic, while others are hydrophilic. The hydrophilic species generally work within the cell to break down excess concentrations of intracellular ACh. The lipidlinked (hydophobic) varieties, however, are the primary agents of ACh inactivation, working at the synaptic cleft or the neuromuscular junction to break ACh into its component parts: acetate and choline. Lipid-linked species of AChE are embedded within the post-synaptic membrane and placed strategically close to the post-synaptic receptor molecules in order to ensure quick inactivation of ACh. Though the individual chemical qualities and anatomical arrangements vary widely between the various forms of AChE, the mechanism of catalysis for all the species remains strikingly similar. In all cases, the active site of AChE is made up of two subsites, both of which are critical to the breakdown of ACh. The anionic site serves to bind a molecule of ACh to the enzyme. When first discovered, this site was thought to be made up of one or more negatively charged groups which electrostatically interacted with the positively charged quaternary nitrogen of the ACh molecule. More recently, however, it is thought that hydrophobic interactions are equally, if not more important in binding this region of the substrate to the enzyme. Once the ACh is bound, the hydrolytic reaction occurs at a second region of the active site called the esteratic subsite. Here, the ester bond of ACh is broken, releasing acetate and choline. Choline is then either temporarily trapped within the junctional folds of the muscular endplate or immediately taken up again by the high affinity choline uptake (HACU) system on the presynaptic membrane. Acetate, however, becomes covalently bonded to serine residues within the esteratic subsite, forming a temporary acetylated form of AChE. A molecule of water then reacts with this intermediate, liberating the acetate group, which diffuses into the surrounding medium. What remains is an active form of the enzyme, ready to react with a newly released molecule of ACh.
Вам также может понравиться
- Neurochemistry LabДокумент13 страницNeurochemistry LabAdel KridОценок пока нет
- Muscle RelaxantsДокумент49 страницMuscle RelaxantsApriani MargastutieОценок пока нет
- NEUROMUSCULARДокумент29 страницNEUROMUSCULARshikhaОценок пока нет
- WWW - plantarchives.org:SPL ISSUE SUPP 2,2019:237 (1347-1359)Документ13 страницWWW - plantarchives.org:SPL ISSUE SUPP 2,2019:237 (1347-1359)Arashmeet kaurОценок пока нет
- Table I Nicotinic and Muscarinic Receptors and Their ActionsДокумент3 страницыTable I Nicotinic and Muscarinic Receptors and Their ActionsMegha Kulkarni FPS PES UniversityОценок пока нет
- Neuromuscular PhysiologyДокумент6 страницNeuromuscular PhysiologySuresh KumarОценок пока нет
- Parasympathomimetic DrugsДокумент15 страницParasympathomimetic DrugsRohit PrajapatiОценок пока нет
- Pharmacology of TheДокумент21 страницаPharmacology of TheKenneth NuñezОценок пока нет
- Cholinergic Receptor Drugs Nicotinic ReceptorsДокумент30 страницCholinergic Receptor Drugs Nicotinic ReceptorsAlee Iz HarОценок пока нет
- Neuromuscular Blocking AgentsДокумент26 страницNeuromuscular Blocking Agentsblessing akataОценок пока нет
- Biokimia SarafДокумент41 страницаBiokimia SarafSang Aji Samudra AnugrahОценок пока нет
- Cholinergic DrugsДокумент44 страницыCholinergic Drugskhuzaima9100% (1)
- Ref Arc Neurotrans Ach NorEPI Cholinergic TransmissionДокумент18 страницRef Arc Neurotrans Ach NorEPI Cholinergic TransmissionAlee Iz HarОценок пока нет
- Control and Coordination PDFДокумент14 страницControl and Coordination PDFmobasser sikderОценок пока нет
- PHARMACOLOGY LECTURES CHOLINERGIC MECHANISMS DR THERESA JOHN Sent To BSC SEP 20 2021Документ33 страницыPHARMACOLOGY LECTURES CHOLINERGIC MECHANISMS DR THERESA JOHN Sent To BSC SEP 20 2021Ebenezer SamuelОценок пока нет
- Alpha7 Nicotinic Acetylcholine Receptor Is A Target in Pharmacology and ToxicologyДокумент20 страницAlpha7 Nicotinic Acetylcholine Receptor Is A Target in Pharmacology and ToxicologyJean Pierre Chastre LuzaОценок пока нет
- CAPE Nervous Coordination NotesДокумент24 страницыCAPE Nervous Coordination NotesSharina GeraldОценок пока нет
- Bio 336 Physio Exam 1 EssaysДокумент3 страницыBio 336 Physio Exam 1 EssayssheilaОценок пока нет
- Depolarizing Muscle RelaxantДокумент40 страницDepolarizing Muscle RelaxantMohammad HayajnehОценок пока нет
- Neuromuscular TransmissionДокумент45 страницNeuromuscular TransmissionparuОценок пока нет
- Tell Them What You're Going To Tell Them. Tell Them. Tell Them What You Just Told Them. AristotleДокумент17 страницTell Them What You're Going To Tell Them. Tell Them. Tell Them What You Just Told Them. AristotleSafiya JamesОценок пока нет
- Muscle ElectrophysiologyДокумент2 страницыMuscle ElectrophysiologyAurelia AlexandraОценок пока нет
- 7 - CholinergicsДокумент10 страниц7 - CholinergicsRaunaq Singh RatraОценок пока нет
- Neuromuscular Junction (NMJ) : Acetylcholinesterases 551Документ8 страницNeuromuscular Junction (NMJ) : Acetylcholinesterases 551Brahmatheja ReddyОценок пока нет
- NeurotransmittersДокумент6 страницNeurotransmitterssuweino_harunОценок пока нет
- A Muscle RelaxantДокумент19 страницA Muscle RelaxantIndah GitaswariОценок пока нет
- Coursematerial 150Документ67 страницCoursematerial 150Endar Wahyu SetiawanОценок пока нет
- 11) Muscle Relaxants - Neuromuscular Junction and PhysiologyДокумент1 страница11) Muscle Relaxants - Neuromuscular Junction and Physiologykarim hassanОценок пока нет
- Course 12 - NeurotransmitersДокумент40 страницCourse 12 - NeurotransmitersPopa NicuОценок пока нет
- Drugs and The BrainДокумент7 страницDrugs and The Brainebola123Оценок пока нет
- Section 2 NeurophysiologyДокумент3 страницыSection 2 NeurophysiologyZirah Lee ValledorОценок пока нет
- Cholinergic DrugsДокумент44 страницыCholinergic DrugsJohn MahiyaОценок пока нет
- Anticholinesterases and Anticholinergic Drugs: V Priya Nair MB Bs Frca Jennifer M Hunter MB CHB PHD FRCAДокумент5 страницAnticholinesterases and Anticholinergic Drugs: V Priya Nair MB Bs Frca Jennifer M Hunter MB CHB PHD FRCAMarinaОценок пока нет
- Neurophysiology, Neurotransmitters and The Nervous SystemДокумент69 страницNeurophysiology, Neurotransmitters and The Nervous SystemKUNNAMPALLIL GEJO JOHN100% (1)
- Synapses and DrugsДокумент16 страницSynapses and Drugssoyoung jinОценок пока нет
- Neuromuscular JunctionДокумент16 страницNeuromuscular JunctionSamadshahirОценок пока нет
- Autonomics Nervous SystemДокумент23 страницыAutonomics Nervous SystemAmarnath SahОценок пока нет
- Neuromuscular JunctionДокумент6 страницNeuromuscular Junctiondrahmed1028Оценок пока нет
- The Neuromuscular Junction (Neuromuscular Synapse) Dr. Taha Sadig AhmedДокумент38 страницThe Neuromuscular Junction (Neuromuscular Synapse) Dr. Taha Sadig AhmedAnil KumarОценок пока нет
- Anticholinesterases and Anticholinergic Drugs: V Priya Nair MB Bs Frca Jennifer M Hunter MB CHB PHD FRCAДокумент5 страницAnticholinesterases and Anticholinergic Drugs: V Priya Nair MB Bs Frca Jennifer M Hunter MB CHB PHD FRCAAbubakar JallohОценок пока нет
- Excitation of Skeletal MuscleДокумент60 страницExcitation of Skeletal MuscleVanessa Mae EncarnadoОценок пока нет
- Neuromuscular PhysiologyДокумент44 страницыNeuromuscular PhysiologyAnkita NigamОценок пока нет
- Synapse & NeurotransmissionДокумент21 страницаSynapse & Neurotransmissionزين العابدين محمد عويشОценок пока нет
- HPHY 225 Assignment - FinalДокумент5 страницHPHY 225 Assignment - FinalSylvester MathiasОценок пока нет
- TetanusДокумент106 страницTetanusEster PadangОценок пока нет
- Acetylcholinesterase Inhibitors From Plants and Fungi: Peter J. Houghton, Yuhao Ren and Melanie-Jayne HowesДокумент19 страницAcetylcholinesterase Inhibitors From Plants and Fungi: Peter J. Houghton, Yuhao Ren and Melanie-Jayne HoweslyndaОценок пока нет
- CH 3b Synaptic TransmissionДокумент31 страницаCH 3b Synaptic TransmissionWOw WongОценок пока нет
- Somatosensory FaalДокумент1 страницаSomatosensory Faalamalia khusnaОценок пока нет
- Congenital Myasthenic Syndromes - Symptoms, Causes, Treatment - NORDДокумент27 страницCongenital Myasthenic Syndromes - Symptoms, Causes, Treatment - NORDFlavia BertolozziОценок пока нет
- CH5 THE SYNAPSE AND ACTION POTENTIAL GENERATION ON THE POSTSYNAPTIC MEMRANE NewДокумент4 страницыCH5 THE SYNAPSE AND ACTION POTENTIAL GENERATION ON THE POSTSYNAPTIC MEMRANE NewoguzОценок пока нет
- SynapseДокумент45 страницSynapseroyjason4518Оценок пока нет
- Acetylcholine: Paul M SalvaterraДокумент7 страницAcetylcholine: Paul M SalvaterraFrancisco BecerraОценок пока нет
- How Neurons Communicate To Each Other at A Chemical Synapse?Документ5 страницHow Neurons Communicate To Each Other at A Chemical Synapse?stepkim92Оценок пока нет
- Chapter 7 Synaptic Transmission 2014 Clinical NeuroscienceДокумент9 страницChapter 7 Synaptic Transmission 2014 Clinical NeuroscienceMarta Casals CollОценок пока нет
- Drugs Affecting The ANSДокумент95 страницDrugs Affecting The ANSKhalid OmarОценок пока нет
- Week5 3 Neurophysiology Part2 2023Документ43 страницыWeek5 3 Neurophysiology Part2 2023yanikashahОценок пока нет
- Pocket - Companion - To - Guyton - and - Hall - Textbook - of - Medical - Physiology - ExportedДокумент7 страницPocket - Companion - To - Guyton - and - Hall - Textbook - of - Medical - Physiology - Exportedhassnfiras04Оценок пока нет
- BIO 361 Neural Integration LabДокумент5 страницBIO 361 Neural Integration LabnwandspОценок пока нет
- Handbook: For Clinical Management of DengueДокумент124 страницыHandbook: For Clinical Management of DengueraattaiОценок пока нет
- 21 An Discharge CriteriaДокумент13 страниц21 An Discharge CriteriaFajr MuzammilОценок пока нет
- Exper Ential Is MДокумент7 страницExper Ential Is MFajr MuzammilОценок пока нет
- Jns 2 39Документ5 страницJns 2 39Fajr MuzammilОценок пока нет
- Annsurg00185 0122Документ6 страницAnnsurg00185 0122Fajr MuzammilОценок пока нет
- 21 An Discharge CriteriaДокумент13 страниц21 An Discharge CriteriaFajr MuzammilОценок пока нет
- Aldrete ScoreДокумент33 страницыAldrete ScoreIndra PutraОценок пока нет
- Distal Splenorenal Shunt: J. Michael HendersonДокумент11 страницDistal Splenorenal Shunt: J. Michael HendersonFajr MuzammilОценок пока нет
- Hepatitis BДокумент18 страницHepatitis BFajr MuzammilОценок пока нет
- Pneumonia 1Документ12 страницPneumonia 1Mohammad Izza Naufal FikriОценок пока нет
- Modul Hematoimun (Dr. Fakhri) - Immunity To Parasitic InfectionsДокумент17 страницModul Hematoimun (Dr. Fakhri) - Immunity To Parasitic InfectionsFajr Muzammil100% (1)
- At Commands For CDMA Modems Incl Qualcomm U300Документ195 страницAt Commands For CDMA Modems Incl Qualcomm U300crisp123Оценок пока нет
- Marshalloy MQ 4142 PHДокумент3 страницыMarshalloy MQ 4142 PH123vigenОценок пока нет
- Cement Lecture Note - AAIДокумент27 страницCement Lecture Note - AAINafizОценок пока нет
- Nucleic AcidДокумент28 страницNucleic AcidStellar OutputsОценок пока нет
- Research Paper 2Документ45 страницResearch Paper 2SushmaSahuОценок пока нет
- Name Roll Number Department Subject: CeramicДокумент5 страницName Roll Number Department Subject: CeramicMohsin AkbarОценок пока нет
- AIIMS 2019 Chemistry Sample Question PaperДокумент10 страницAIIMS 2019 Chemistry Sample Question PapermisostudyОценок пока нет
- Science PYQДокумент82 страницыScience PYQgovidaswamygОценок пока нет
- TCP I Two Marks Question With Answer Unit IiДокумент12 страницTCP I Two Marks Question With Answer Unit IiJana MuthuОценок пока нет
- MINERALSДокумент90 страницMINERALSlyjohnjoel maglacasОценок пока нет
- Envisci Quiz 3Документ3 страницыEnvisci Quiz 3Ysabel Apostol100% (1)
- Cement, Gypsum Product, Quartz SandДокумент14 страницCement, Gypsum Product, Quartz SandHuda FauziОценок пока нет
- Modern Drymix Mortars: Performance Additives ForДокумент15 страницModern Drymix Mortars: Performance Additives Form_shahbaghiОценок пока нет
- Sodium Metal AnalysisДокумент2 страницыSodium Metal AnalysisCindy GallosОценок пока нет
- Hydro CarbonsДокумент10 страницHydro CarbonsLok Jun Hao100% (1)
- 19660004844Документ244 страницы19660004844akshukОценок пока нет
- Ss1 Biology. 2Документ5 страницSs1 Biology. 2Enchalew Shitaye100% (1)
- Role of Solvent in SNДокумент9 страницRole of Solvent in SNsarahОценок пока нет
- Ibuprofen (Pharmacopoea)Документ3 страницыIbuprofen (Pharmacopoea)Titis Adisti HapsariОценок пока нет
- IGCSE - Air and WaterДокумент93 страницыIGCSE - Air and WaterJashan Rohit KumarОценок пока нет
- Safety Data Sheet: According To EC Directive 91/155/EECДокумент6 страницSafety Data Sheet: According To EC Directive 91/155/EECTitiОценок пока нет
- Conservacion de FosilesДокумент9 страницConservacion de Fosiles2304480Оценок пока нет
- Definitions - Chemistry IAL EdexcelДокумент7 страницDefinitions - Chemistry IAL EdexcelPanagiotis ScordisОценок пока нет
- Wastewater Treatment Using DAF For Process Water Reuse in Apatite FlotationДокумент7 страницWastewater Treatment Using DAF For Process Water Reuse in Apatite FlotationYessica Botero VargasОценок пока нет
- Taller Quimica IДокумент35 страницTaller Quimica IZantiiagoo TrujilloОценок пока нет
- Polimerization PPДокумент4 страницыPolimerization PPidgenОценок пока нет
- Organic Name Reaction List For IIT - NEETДокумент3 страницыOrganic Name Reaction List For IIT - NEETmolakathallanehareddyОценок пока нет
- Cudahy - 2017 - Regional To Prospect Scale Exploration For Porphyry-Skarn-Epithermal Mineralisation at Yerington, ASTER and Airborne Hypersp PDFДокумент27 страницCudahy - 2017 - Regional To Prospect Scale Exploration For Porphyry-Skarn-Epithermal Mineralisation at Yerington, ASTER and Airborne Hypersp PDFRoberto MoraОценок пока нет
- 5 bookokEDITULANGrevisiJojoJurnal4 PDFДокумент15 страниц5 bookokEDITULANGrevisiJojoJurnal4 PDFYoga PratamaОценок пока нет
- Notes - Separation of SubstancesДокумент4 страницыNotes - Separation of SubstancesJumayma MaryamОценок пока нет
- CarbohydratesДокумент112 страницCarbohydratesmaryam ijazОценок пока нет
- Why We Die: The New Science of Aging and the Quest for ImmortalityОт EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityРейтинг: 4 из 5 звезд4/5 (3)
- Gut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)От EverandGut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)Рейтинг: 4 из 5 звезд4/5 (378)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisОт EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisРейтинг: 3.5 из 5 звезд3.5/5 (2)
- Gut: the new and revised Sunday Times bestsellerОт EverandGut: the new and revised Sunday Times bestsellerРейтинг: 4 из 5 звезд4/5 (392)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincОт EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincРейтинг: 3.5 из 5 звезд3.5/5 (137)
- A Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsОт EverandA Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsРейтинг: 4.5 из 5 звезд4.5/5 (6)
- The Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceОт EverandThe Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceРейтинг: 4.5 из 5 звезд4.5/5 (517)
- 10% Human: How Your Body's Microbes Hold the Key to Health and HappinessОт Everand10% Human: How Your Body's Microbes Hold the Key to Health and HappinessРейтинг: 4 из 5 звезд4/5 (33)
- All That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesОт EverandAll That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesРейтинг: 4.5 из 5 звезд4.5/5 (397)
- Tales from Both Sides of the Brain: A Life in NeuroscienceОт EverandTales from Both Sides of the Brain: A Life in NeuroscienceРейтинг: 3 из 5 звезд3/5 (18)
- Fast Asleep: Improve Brain Function, Lose Weight, Boost Your Mood, Reduce Stress, and Become a Better SleeperОт EverandFast Asleep: Improve Brain Function, Lose Weight, Boost Your Mood, Reduce Stress, and Become a Better SleeperРейтинг: 4.5 из 5 звезд4.5/5 (15)
- The Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorОт EverandThe Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorОценок пока нет
- Human: The Science Behind What Makes Your Brain UniqueОт EverandHuman: The Science Behind What Makes Your Brain UniqueРейтинг: 3.5 из 5 звезд3.5/5 (38)
- The Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionОт EverandThe Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionРейтинг: 4 из 5 звезд4/5 (812)
- Undeniable: How Biology Confirms Our Intuition That Life Is DesignedОт EverandUndeniable: How Biology Confirms Our Intuition That Life Is DesignedРейтинг: 4 из 5 звезд4/5 (11)
- Who's in Charge?: Free Will and the Science of the BrainОт EverandWho's in Charge?: Free Will and the Science of the BrainРейтинг: 4 из 5 звезд4/5 (65)
- The Rise and Fall of the Dinosaurs: A New History of a Lost WorldОт EverandThe Rise and Fall of the Dinosaurs: A New History of a Lost WorldРейтинг: 4 из 5 звезд4/5 (595)
- Seven and a Half Lessons About the BrainОт EverandSeven and a Half Lessons About the BrainРейтинг: 4 из 5 звезд4/5 (109)
- Good Without God: What a Billion Nonreligious People Do BelieveОт EverandGood Without God: What a Billion Nonreligious People Do BelieveРейтинг: 4 из 5 звезд4/5 (66)
- Moral Tribes: Emotion, Reason, and the Gap Between Us and ThemОт EverandMoral Tribes: Emotion, Reason, and the Gap Between Us and ThemРейтинг: 4.5 из 5 звезд4.5/5 (115)
- Buddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomОт EverandBuddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomРейтинг: 4 из 5 звезд4/5 (216)
- A Series of Fortunate Events: Chance and the Making of the Planet, Life, and YouОт EverandA Series of Fortunate Events: Chance and the Making of the Planet, Life, and YouРейтинг: 4.5 из 5 звезд4.5/5 (62)
- The Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindОт EverandThe Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindРейтинг: 4.5 из 5 звезд4.5/5 (93)
- Return of the God Hypothesis: Three Scientific Discoveries That Reveal the Mind Behind the UniverseОт EverandReturn of the God Hypothesis: Three Scientific Discoveries That Reveal the Mind Behind the UniverseРейтинг: 4.5 из 5 звезд4.5/5 (52)
- Lymph & Longevity: The Untapped Secret to HealthОт EverandLymph & Longevity: The Untapped Secret to HealthРейтинг: 4.5 из 5 звезд4.5/5 (13)