Академический Документы
Профессиональный Документы
Культура Документы
CR-CG Paradox Resolution 4
Загружено:
LE PathwayОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
CR-CG Paradox Resolution 4
Загружено:
LE PathwayАвторское право:
Доступные форматы
Resolving Apparent Paradoxes of AKT, AMPK, TOR, PGC1alpha and SIRT 1 Regulation in Growth/Aging Metabolism
Gregory S. Bambeck Ph.D. and Warren Weller H. B. Summary In the eukaryotic animal cell, the cell growth (CG) and caloric restriction (CR) metabolic control element pathways basically run in opposition to one another. This includes the up and down activation of their regulatory control proteins such as AKT, AMPK, TOR, PGC 1alpha, SIRT 1 and others. An activated CG pathway is a relatively straightforward and explosive affair with mostly unambiguous drive states in the presence of adequate nutrient fuel. Conversely, the CR drive state, characterized by nutrient fuel paucity, bioenergetic ATP and redox NADH deficiency and the extreme need for elevated energy production efficiency, seems to have elements of conflict and paradox within its own life extending central CR drive state, and is more circumspect and confusing than CG. When viewed from the perspective that the CR system contains requisite SIRT 1 periodic mini-CG drive states, many of these conundrums seem to resolve. We tie this notion to evolution by noting that the CG system still retains many of the phosphate dominating metabolite flux features of anaerobiasis, which preceded aerobic acetyl activating metabolite fluxes by over a billion years on earth. The mitochondrion, an aerobic symbiont become organelle, may have been originally employed by its host to generate ATP during times of frugality in exchange for safety, and still retains both phosphate and acetyl manipulated remnants of anaerobic subordinating regulatory functions, as manifest with remarkably little fundamental component change, from yeast to human. Including the evolutionary perspective adds contextual meaning to the logic of regulatory element conflict resolution, the diseases of aging and life extension.
Introduction Throughout evolution, the living cell has been charged with the mission of retaining energetic ATP/AMP and redox NAD/NADH homeostasis under either gain of mass cell growth (CG), loss of mass caloric restriction (CR) or nutrient non-restricted steady state entropic disequilibriums. Herein, we focus primarily on the CG and CR regulatory control systems. Early in the twentieth century, it was established that mice have extended life expectancy considerably beyond the normal known limit when subjected to a rather severe about 40% of ad libitum caloric restriction. Later, this phenomenon was shown to be manifest, in what appears to be, the full range of proto-animalia to multi-cellular animalia from yeast fungus, to worms, insects, spiders, and up the vertebrate tree from fish, through mammals and the primate branch. Our discussion will mostly center upon mammalian cell physiology. Later experiments demonstrated that chronically enforced cell growth shortened life expectancy. At the turn of the twenty-first millennium, it emerged that the CG and CR systems are metabolic regulatory control pathways whose inputs oppositionally converge on target of rapamycin (mTORC1, in mammals, hereafter called TOR), from which, oppositional outputs affected a wide range of differential anabolic and catabolic transcription gene sets. On July 23, 2011, we published a basic regulatory metabolic flow chart (map) that outlines the oppositional CG and CR metabolic regulatory systems as a direct, simple and straightforward dynamic singularity, albeit as a primary skeletal framework into which, system modifiers can be integrated. In that publication, we give a fairly detailed description of the workings of the flow chart. We also describe the processes in the flow chart in terms of normal and diseased conditions, as well as from the life extension perspective. Further, we list selected food supplements with mechanisms of action impacting the CG and CR pathways that have medical benefit from a life extension and diseases of aging standpoint. The full and unabridged textual description of this flow chart is found at the publication house SCRIBD, is entitled Life Extension Metabolic Pathway Map
Reveals New Phytonutrient Candidates, and can be single step accessed through the internet via www.facebook.com/lepath. For those newly introduced to the CG/CR life extension metabolic pathway system, it might be helpful to read our previous publication, as an adjunct to this narrative. For those not so inclined, or for those better versed, we provide the flow chart with a modified and abbreviated description, as a courtesy, below. CG/CR Pathway Map and Review The CG and CR metabolic pathway map is shown below. The CG pathway is principally activated by survival factors and cell growth factors through protein kinase cascades that converge upon and activate AKT as their common focal point when nutrient fuel (particularly glucose) is plentiful. This leads to an up regulation of the major metabolic control switch called TOR. In the CG drive state, TOR activates a wide range of anabolic and anabolic support processes that couple nutrient fuel and cell building block metabolite importation to the anabolic systems that build a new cell. This necessitates the catabolic making of ATP for energy requiring anabolism, NADH and NADPH for anabolic reduction and free radical (ROS) defusing, in addition to macromolecular, microbody, microsomal and organelle assembly, a full round of DNA replication etc. Most all of these CG bioenergetic and redox needs are paid for from the catabolic machinery manipulations of anaerobic glycolysis and aerobic mitochondrial functions. Under the auspices of the phosphorylation cascade CG drive state, considerable alterations take place in the mechanistic operations, both in type and degree, between the very ancient anaerobic glycolytic pathway and the less ancient mitochondrial Krebs cycle linked respiratory/OX/PHOS system. Under the CG drive state, glucose importation is dramatically up regulated, while the mitochondrial respiratory chain becomes less efficient and OX/PHOS becomes more uncoupled, which in turn, creates a ROS uptick. On the surface of it, particularly from the production of ATP standpoint, this seems counterintuitive as steady state oxidative mitochondrial ATP production is about ten times as productive, on a per glucose basis, as glycolysis. Elevated glycolytic rate, however, increases ten (to a hundred-fold in extreme cases) and increases
anaerobic ATP production (by a smaller multiple than its glycolytic rate increase) to accomplish a three-fold primary function: 1) it changes the glycolytic to mitochondrial ATP production ratio, 2) increases the cellular ATP/AMP ratio, and 3) shunts glycolytic intermediates through the pentose phosphate pathway (PPP). This third item provides pentose for nucleotide production, phosphorylated substrates for anaerobic substrate phosphorylation energy carriers, substrates for fatty acid triesterification and NADPH for reducing and ROS defusing power. The glycolytic end product lactate to pyruvate ratio is shifted upward, which increases the glycolytic end product export waste product to Krebs cycle feedstock acetyl CoA ratio. All of these ratios are very important to metabolite flux and its outcomes.
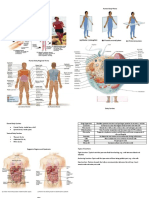
Figure 1. Unified cell growth (CG) and caloric restriction (CR) metabolic map.
In addition, the CG drive state activates hypoxia inducible factor (HIF) to stimulate glycolysis via direct glycolytic pathway enzyme and fetal pyruvate kinase 2 (PKM2) gene transcription, to depress respiratory OX/PHOS via the mitochondrial retrograde pathway (RTG) and to accelerate the PPP. It also activates vascular endothelial growth factor (VEGF) to induce angiogenesis and the inflammatory cytokine cascade, to prepare for CG induced replicative tissue invasion. It is noteworthy that this shift occurs under normoxic or hypoxic conditions in mammalian cells by the same control pathways found in yeast when manually shifted from aerobic to fermentation growth conditions. Reams of papers are emerging that attest to the modern regulatory renaissance understanding of differential catatabolisms more primitive Warburgian roots. A real clean and very recent paper, that measures all the variables via the inositol phosphates IP6 and IP7 CG down regulators by Z. Szijgyarto et. al., Science vol. 334, p.802, 11/11/2011, demonstrates this elegantly in both yeast and mouse embryonic stem cells. They measured ATP/AMP, NAD/NADH, glycolytic rate and mitochondrial respiration, OX/PHOS rates and coupling, in both the IP6-IP7 up and down regulated states and found all of the appropriate aforementioned affects in type, quantity and sequence. It was the IP7 which was the new kid on the block, as IP6 is a well known AKT inhibition driver. Not too surprisingly, IP7 seems to mostly act as an accelerated version of IP6, to work in concert with ATP energy demands, and then is rapidly cycled back to IP6. Inhibition of AKT has been associated with increased life expectancy and decreased incidence and severity of diseases of aging, such as cancer, diabetes neurodegeneration, muscular wasting and atherogenic cardiovascular disease. Simultaneous up regulation of the CR pathway has the same medical benefits and is synergistic with CG down regulation of AKT pathway drivers. On the aerobic side of the equation, CG activated TOR phosphorylates PGC 1alpha, which in turn, activates the mitochondrial biogenesis program. PGC 1alpha activates nearly the whole of the gene transcription set for making new mitochondria. As we have seen, CG activated mitochondria are respiration and OX/PHOS deficient. This is due to the fact that new mitochondria lack a few respiratory proteins whose 4E-BP activated late respiratory gene transcripts are stimulated when a deactivated TOR shuts down PGC 1alpha biogenesis. For this reason, we (the authors) call the first incomplete phase neogenesis and the
second completing phase regenesis. Also under CG, mitochondrial uncoupler proteins (UCP) are activated and the chemiosmotic mitochondrial outer membrane potential (MOMP) becomes compromised. By now, it should be obvious to the reader why this neogenic vs. regenic distinction is critical, as the CG system is dependant upon it for controlling substrate flow and bioenergetic and redox dynamics. Throughout CG, HIF also assists in inhibiting apoptosis, as cytochrome c leakage during MOMP collapse can sensitize and even activate apoptosis. Chemotherapeutic mitochondrial MOMP depressed enhancement in cancer cells can be medically advantageous by driving apoptosis, as was very recently demonstrated by T.N. Chonghaile et.al. in Science vol.34, p.1129, 11/25/2011. How the excess MOMP deficient freed protons are apportioned under CG conditions, and for what purpose beyond uncoupling, is a mystery. Do they assist in anabolic reduction in the cytoplasm? Under CG conditions, mitochondrial replication rate undergoes an explosive increase. In the matter of several hours, a cell may produce the equivalent of several hundred mitochondria as an interstitial continuum or as individual mitochondria. During a rest phase between rounds of replication, regenesis may complete the mitochondria. When rounds of replication are rampantly chained in a continuous sequence, as in early embryogenesis or in rapidly growing cancers, mitochondria remain in a chronic state of neogenic incompleteness. Although cancer cell mitochondria are not often defective, what causes them to operate as if defective is often itself, defective. Regardless of functional origin, the net outcome is virtually identical, and cries for elucidation over argument. Such argumenative sophistry, starting in the mid 1950s and finalizing in the early 1970s, more than just upended OttoWarburgs and others careers, and set intermediary metabolic theory back no less than a good thirty years, as pointed out in a multiple article topic focus set in Science, vol.330, p.1337-1358, 12/03/2010. Mutagenically induced regulatory defects in cancer cells can cause their mitochondria to remain stuck in a neogenic state, even during slow growth, while normal cells eventually play regenic catch-up when the CG and
replication programs slow down or shut down. A multiplicity of major CG and CR regulators, especially p53 tumor suppressor and RAS, are mutated in a clear majority of cancers that, in turn, demonstrate activated glycolysis, and quite often, a mitochondrial MOMP deficit, respiratory system I and system III component impoverishment and/or UCP activated uncoupling. Recently, glycolytic disruptors and rectifiers, as well as glycolysis to mitochondria interface anti-cancer therapies have also shown enhanced kill or growth rate depression merit, in addition to the aforementioned assisted MOMP decay therapy. The CR side of the metabolic map is best described via depleted exogenous nutrient fuel importation elevated AMP activation of AMPK and the eventual AMPK pathway down regulation of TOR, which halts anabolism and begins a wholesale transition in anaerobic and aerobic activity and metabolite flux. Glycolysis slows down and pyruvate becomes just about its only carbon output, which is converted to acetyl CoA as a Krebs cycle input, as the PPP is minimalized. The down regulated TOR deactivation of PGC 1alpha shuts down mitochondrial neogenesis, and mitochondrial regenesis is activated to make tightly coupled efficient use of scarce fuel and to minimize dangerously toxic ROS production. This particular action can be reinforced through a synergistic AMPK activation by p53 tumor suppressor protein up regulation via the SESN ROS detector protein. Upon TOR down regulation, PGC 1 alpha shifts to its other active state. It associates with peroxisome proliferator coactivator receptor protein isoforms (PPAR gamma, mostly), to enhance lipid catabolism as a compensation for the falling off of glucose importation. This provides fat derived acetyl CoA production as a mitochondrial feedstock. It is no wonder that acetic acid, in addition to being a next step after glycolytic output, is also a mitochondrial input from either sugar or fat, is a major regulator of PGC 1alpha, which is a protein festooned with acetylation/deacetylation sites, and whose two major activated states focus on the most core of all mitochondrial needs: i.e. acquiring a principal energy feedstock and even existing in the first place. Apparently, the silent information repeat protein (SIRT 1) can deacetylate all thirteen of the PGC 1alpha lysine sites and a newly found AKT CG site during CR, and has thus, become a source of pathway activation contention, as we
describe in the next section. SIRT 1 is conspicuously absent from our direct impact CG and CR map, and for good reason, as we shall also see. Lastly, when CG is inhibited and CR is activated, the whole HIF, VEGF and inflammatory cascade are shut down. Under CR conditions the autophagic recycling of defective cell parts is up regulated. Hopelessly defective cells are removed via initiation of apoptotic cell suicide, but only in the event of autophagic rescue failure. CR induced autophagy normally inhibits the apoptotic program, as does the previously described CG activated MOMP deficiency state, and with HIF and its glycolytic RTG response feedback relationship with mitochondria. There seem to be phosphate and acetate elements of evolutionary holdover in the CG vs. CR regulatory system. On the CG side of the system, phosphatases and kinases dominate most activating and repressing functions. They even radiate upward from AKT to the whole of the branching CG factor input cascade, and funnel downward and re-branch to the glycolytic, PPP CG support, anabolic and mitochondrial neogenesis networks. Anaerobic substrate phosphorylation itself evolved as a bioenergetic alternative and synthetic support network attendant for, from and to, ATP. This makes thermodynamic in addition to evolutionary sense, as hydroxylated aldo and keto carbons and their energy rich 3 and 6 carbon phosphates were the currency in trade for glycolysis and the PPP anabolism setup prior to planetary oxygen availability. Even the Krebs cycle is technically an anaerobic hydration and dehydrogenation process, as a kind of real fancy and orchestrated downstream substrate phosphorylation form of glycolysis, probably even predating the respiratory/OX/PHOS system connections, but thats another heavy duty evolutionary discussion. Alternatively, acetylation and deacetylation protein regulation, at least from a metabolic standpoint, seem to be more apparent during CR and are tilted toward aerobic functional control, as a holdover from its principle feedstock molecule, acetic acid. Of course, these functions have become mixed over time as the two systems have become so inextricably tied to one another, but more than just inklings of the remnants remain. This may become more evident when we include new and surprising SIRT 1, AKT and TOR function discoveries.
The straightforward and simplistic interpretation of the oppositional manifestation of the CG and CR pathways and their differential application lend credence to the notion that anaerobic to aerobic molecular ontogeny remnant functions are still detectable in modern organisms. This is somewhat evident if one just compares the metabolite molecular structures themselves. However, recent second tier regulatory findings demonstrate that the direct and simplistic interpretation of the CR pathway results in some apparent conflicts and paradoxes. Our anaerobic to aerobic remnant interpretation adds a layer of complexity to the simplistic interpretation, by including the addition of circumstance and periodic timing to the mix. As we hope to show next, our more complex hypothesis might resolve some of the conundrums we are about to preview and then, articulate. CG vs. CR Regulatory Paradoxes and Resolutions As a skeletal framework, the metabolic pathway map appears rather coherent and consistent in the large view, especially when one considers that the mechanism of action of all of the known and putative life extension CR mimetic molecules down regulate AKT or up regulate AMPK to inhibit TOR, or inhibit TOR directly, just as does CR itself. However, when one scrutinizes beyond the maps overarching structural outline to include actual cytological operational subroutines, the apparent conflicts and paradoxes begin to emerge. Although the apparent inconsistencies are few in number, the fact that they involve the major regulatory proteins renders them quite significant. For instance, PGC 1alpha, primary director of mitochondrial biogenesis (neogenesis), is turned on explosively during CG, often generating several hundred mitochondria in just a few hours. Conversely, PGC 1alpha is down regulated during CR onset and general maintenance, but over the long haul, the creation of new mitochondria of the regenic function type, occurs slowly and relentlessly under even fairly severe CR; for instance, taking several months in humans. Another intellectual irritant arises from the fact that in some papers, PGC 1alpha is up regulated during CG, while in other papers it is up regulated during CR, and with opposing effects. Depending on what pathway is the focus topic of the paper, one scientists activation is another scientists inhibition. A
common contextual lexicon would help us to know when PGC 1alpha does something, what it does when it does it, and how all of that integrates with what is meant by excitation or inhibition. Similarly, SIRT 1, once considered critical to life extension, is fraught with scientific conflicts, as will be discussed later. Even AKT, TOR and AMPK, CG and CR mainstays, are not immune from the fracas, although their principal functions remain solidly intact. Many papers stray from target molecule mechanisms of action to include multi-step downstream pathway affects. These may or may not turn out to be wholly true, as other anastamosing regulatory systems can intervene and bungle the apparent appearances of cause and effect connectedness. There are just too many hundreds of references to choose from to illustrate these points. This often happens when a hot topic takes on all the trappings of a gold rush. As a result, reading too many of them will just about drive you crazy. It does not pay to stray too far afield of main effects, albeit stray we must, or hypotheses could not exist. Regardless, the concept of life extension itself and the promise of preventing, ameliorating or curing, the diseases of aging that are killing the vast majority of us, are perceived as such golden apples that research is barreling forward like a runaway freight train. Over the last two years, AKT, AMPK, TOR, PGC 1alpha and SIRT 1 are beginning to be seen more in terms of timing, degree and circumstance as opposed to on vs. off and system A vs. system B type logic, as well articulated by E. H. Jennings et.al. in Oncogene, vol.29, p.1056, 8/19/2010, C. Canto et.al. in Nature, vol.458, p.1056, 4/23/2009 and others. In addition, a freshet of new understandings, as recent as the waning months of 2011, just prior to this publication, are allowing the authors of this narrative to see these regulatory interactions less as a conundrum and more in terms of a time and function harmony. We now turn our attention to the major players and new discoveries. Most of this discussion will center within the CR function, as CG driver states are more robust, straightforward and well understood. However, as we shall see, our analysis will show that the existence and timing of CG subroutines might just be necessary if cells are to survive under CR conditions, and if we are to make sense of the confusion. From a timing perspective, the response of AMPK to the lack of bioenergetic ATP availability via increased AMP concentration is a fast response that can
happen in seconds to many minutes, to even hours depending upon the speed, type and severity of the CR. One may envision cyanide poisoning, an insulin induced hypoglycemic episode or post-prandial sleep or fasting as three time related examples, respectively. Regardless, AMPK response can be fast and is fast when AMP rises, and it is the most pronounced first counter-CR response when external glucose fuel becomes unavailable. As previously described, the AMPK cascade through TOR, CG inhibition via AKT, mitochondrial regenesis etc. all follow. Perhaps, now is the best time to fast forward to the real big problem child, SIRT 1. SIRT1 responds to a much more slowly developing situation in the cell. As the cell shuts down its energy using anabolic systems, readily available internal fuel stores are tapped into and then, eventually used up. At this point the redox potential of the cell begins to collapse and the NAD to NADH ratio rises, which in turn, activates SIRT1 deacetylase. It is important to note that if AMPK is activated at X time units after elevation of AMP/ATP, then the NAD/NADH activation of SIRT 1 may take as long as one to two orders of magnitude longer than X time units. Because of the regular early observations that the AMP/ATP AMPK and NAD/NADH SIRT1 CR activation sets occurred under CR conditions, the life extension research paradigm from the years 2000-2006 was driven by the hunt for AMPK and SIRT 1 activators. The wheels really began to fall off the SIRT 1 bandwagon when direct SIRT 1 activators turned out not to be true life extenders or that many purported SIRT 1 activators were really AMPK activators, AKT deactivators or direct TOR down regulators (such as metformin and rapamycin), with SIRT 1 stimulation appearing to be more of a deeper CR phase automatic outcome of upstream CR master regulators, as demonstrated by Affymetrix gene chip shotgun mRNA population change, and other such as pulse-chase, analyses. The finding that active SIRT1 pro-stimulated AKT by deacetylation by Sundaresan, et. al. Sci. Sig. ra 46, July 2011, and promoted tumor growth just like all CG activators and inhibited tumor growth when absent, pretty much signed SIRT 1s death warrant as a genuine life extender, because it is the down regulation of AKT which is associated with tumor growth inhibition, resistance to diabetes and life extension. However, the notion that SIRT1 might be behaving as a CG subroutine within a
master CR regulatory network, just may find salvation within such an apparent functional antithesis. It is beginning to appear that the earlier findings of SIRT 1 associated CR impacts, such as TSC2 up regulation and TOR inhibition might actually have been time sequence confounded CR to AMPK driven experimental design/conclusion artifacts. The SIRT1 deacetylation and subsequent activation of AKT is consistent with its deacetylation of AMPK phosphorylated PGC 1alpha, as a SIRT 1 to PGC 1alpha sensitizer, and its deacetylating PGC 1alpha activation of mitochondrial neogenesis. Also consistent with a CG function, SIRT 1 dampens the activity of the p53 tumor suppressor protein that activates AMPK, inhibits the p53 anti-ROS gene set and significantly activates AKT. As a more pleasant outcome, SIRT 1 up regulates its own anti-oxidant gene transcription subset alternative to p53 down regulation. One might ask what good it might do to activate a CG system when there is no fuel for accelerated glycolysis or PPP and no importable building blocks for S6K driven anabolism and cell growth. In this mode, SIRT 1 seems to be activating CG mostly along the AKT/TOR/PGC 1alpha axis while not significantly affecting the S6K anabolism and HIF/glycolysis/PPP anabolic support system. Is this an actual regulatory affect or simply a substrate limiting outcome? Another hint may come from the master CG vs. CR mutual toggle switch TOR. Normally, CG driven TOR up regulates HIF, glycolysis, S6K anabolism, mitochondrial neogenesis etc. through the cell growth factor stimulated AKT pathway. A new alternative for TOR activation, via a membrane attached lysosomal internal amino acid abundance detection mechanism is described by R. Zonca et.al. in Science, vol.334, p.678, 11/4/2011. This activating strategy may have some evolutionary aerobic catabolic connection in that it is a lysosomal externally membrane bound ATPase-RAG complex that recruits inactive TOR from the cytoplasm to become Rheb activated when the amino acid stimulated ATPase proton pump acidifies the lysosomal lumen and dissociates the ATPase-RAG complex to activate TOR. This system is surprisingly independent of the CG via AKT/TSC2/TOR pathway, as it can drive lysosomal membrane bound TOR activation in cell and cytoplasm free lysosomal suspensions simply as a result of lysosome amino acid loading and ATPase
activation, alone. This supports a previously published notion that microsomal associated TOR can operate independently of classical CG driven TOR, and further, can be geographically partitioned in the cell. Whether lysosomal TOR actually operates in a CR driven CG subroutine, is too early to tell, but certainly would be a tempting subject for investigation. Lysosomal amino acid loading can occur via extracellular importation or from autophagy. Autophagy is inhibited during CG, while fuel importation is severely constrained during CR. Autophagy and mitophagy are up regulated during CR, particularly when readily available internal food stores run low and internal component recycling becomes an absolute necessity. Autophagy comes to the rescue by raiding defective internal cell resources and by providing nutrient energy rich and metabolic building block loaded autophagosomes that merge with and bud off into lysosomes. It appears that all the correct components have appeared in time, space and circumstance to elicit a cellular house cleaning and efficiency upgrade with scavenged resources, just ahead of a worsening, and possibly, critical famine: everything the cell needs, except for an impossibly unavailable external food supply, that is. Consider the following hypothesis: As CR institutes elevated AMP/ATP, AMPK is activated. Cross pathway regulation inhibits the P13K, AKT, TSC 2 to TOR CG axis. Inhibited TOR shuts down all anabolic S6K, HIF/glycolytic and mitochondrial neogenesis from PGC 1alpha via TOR kinase deactivation while AMPK phosphorylates PGC1 alpha in preparation for SIRT1 deacetylase, which if needed, may come later. TOR deactivation turns on the 4E-BP mitochondrial regenesis program to diminish ROS output and increase ATP production efficiency. PGC 1 alpha switches on its lipid recruitment and lypolysis program to provide for acetyl CoA feedstocks into the Krebs cycle to make up for falling glycolytic input and output and the eventual exhaustion of readily available internal food stores. As stores exhaust, NAD/NADH rises and SIRT1 deacetylation is activated as a CG subroutine of CR, as opposed to classical phosphate activation. SIRT 1 engages a deacetylation AKT, lysosomal TOR to deacetylated PGC 1alpha CG axis, as a punctuated CG subroutine for macromolecular anabolic assembly, and mostly bypasses anabolic metabolite production via the glycolytic and PPP anabolic and redox support pathways,
which, at the very least, suffer from feedstock limitations. SIRT 1 deacetylated PGC 1alpha reactivates the mitochondrial neogenesis program to build a small contingent of new mitochondria from recycled cell parts derived from lysosomal TOR activation(?) through CR initiated autophagy. In addition, SIRT 1 temporarily stunts AMPK and p53 activity and erects its own ROS fighting program in p53s stead. Immediately following the re-establishment of NAD/NADH redox potential, the SIRT1 sub-program shuts down, AMPK reestablishes CR system master control, PGC 1alpha is GCN re-acetylated, 4E-BP is rapidly reactivated (if re activation is even necessary under these circumstances) and thus, mitochondrial regenesis ensues, the ROS count remains low and efficient mitochondrial ATP production assures maximum energy production in combination with minimum fuel utilization. In this scenario, the SIRT 1 subroutine cycles back and forth like a metronome, as needed, within the CR master program. In a sense, the CR machinery turns like a wheel, going from a CR dynamic to a special form of CG sub-routine, and then, back around to a second, and perhaps weaker CR dynamic, again. Round after round of CR-CGCR continues to attempt to retain homeostasis as long as possible with minimal external fuel until the cell either limps along interminably, exhausts itself to death in defect ridden apoptotic starvation or locates exogenous food and breaks the cycle. End of hypothesis. In addition to resolving the metabolic pathway conflicts, this system maintains frugality and helps explain the long term slow gain in mitochondrial numbers with a high efficiency, a result that is also observed with, so called, CR mimetics that either down regulate AKT, up regulate AMPK, or their direct up and down line effectors and effects, respectively. Food supplement extraction enhanced AKT inhibitors, such as genistein, epi-gallo catechin gallate and IP6, and AMPK activators such as resveratrol, curcumin, silibinin and berberine are discussed in our, previously referred to, metabolic map paper. Although such food supplement mechanisms of action are a bit pharmacologically promiscuous, their main effects are quite robust when purified and rendered bioavailable. Our hypothesis is in general agreement with the timing of events and circumstances which occur as the CR condition unfolds. This is merely a hypothesis, in outline, because the actual system contains myriad feed back, feed forward, cross
regulatory etc. subroutines that obviously add finesse to the main bull in a china shop obtuseness of our present knowledge. Concluding Remarks A hypothesis such as this one brings up more metabolic control questions than it resolves. However, if it is even close to correct, it may provide an outline for some hypothesis driven research, as such a need is pined for in Aging Genes: The Sirtuin Story Unravels, by J. C. Frankel, in Science, vol.334, p.1194, 12/2/11. For instance, we only addressed the principal regulatory functions of the main players as we know them today. These molecules have many intermediate modified phosphorylation, acetylation and methylation states that are poorly understood. We have barely begun to scratch the surface of autophagic function and regulation. By what mechanism is it that only a part of a GC system can be turned on, and under what other conditions and for what other purposes? We know quite a bit about this system when under extreme CG and CR drive states, but how are their regulatory elements softer logics integrated under the gentler and more steady state conditions when a well fed organism has stopped growing? No doubt, the evolved armada of major regulatory protein isoforms, are involved, if previous experience with tissue specific isozymes is any guide. For instance, malonyl CoA and succinyl CoA creating demalonylation and desuccinylation lysine site targets of SIRT 5 are all metabolic enzymes, and both these CoAs are among the more common fatty acid metabolism and Krebs cycle intermediates, as recently elucidated by J. Du et. al. in Science, vol.334, p.806, 11/11/2011. It is abundantly clear that we need numerous metabolomic pulsechase experiments to map out the multi-functionality and dynamic timing of events, so as to be able to decipher more meaning. Focused evolutionary tree genomic mapping and proteomic sequences might offer further insight. How the ancient metabolic machinery integrates the billion, or so, year gap between anaerobiasis and aerobiasis and the metabolic regulatory control of the differential gene set activation of the nuclear regulatory controls themselves, and how they feed back to metabolic regulatory orchestration under differing nutrient energy drive states, remains a quandary beyond our present ken. It does appear, however, that metabolically anaerobic proto-eukaryotic life predominantly
utilized mostly phosphate control, and then added more acetate control as aerobiasis became more integrated into its master control system. This was undoubtedly a very lengthy process, as the mitochondrial genome was slowly lost to the mother cell nucleus and histone acetylation/deacetylation gene set transcript regulation became integrated with mitochondrial energy feedstock manipulation, mitochondrial bioenergetic activity and mitochondrial numbers. We propose that much of this became acetylation sub-routines for aerobic metabolic control within the master framework of the pre-existing anaerobic CG and CR systems. Of course, much evolution has happened since the days dominated by the single celled aerobic eukaryote prior to multicellularity, and protein acetylation has branched out to nearly all facets of cell function. However, we believe that its ancient remnant roots are still visible in the major intermediary metabolic regulatory proteins themselves. Gregory S. Bambeck Ph.D., e-mail: gregorybambeck@yahoo.com Warren Weller H.B., e-mail: warren.weller@yahoo.com Copyright by Gregory S. Bambeck Ph.D. and Warren Weller, Dec. 23, 2011
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- A Health and Life Extension Dietary Protocol For Even The Laziest of MinimalistsДокумент3 страницыA Health and Life Extension Dietary Protocol For Even The Laziest of MinimalistsLE PathwayОценок пока нет
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- Glenn Beck SuicideДокумент2 страницыGlenn Beck SuicideLE PathwayОценок пока нет
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Glenn Beck SuicideДокумент2 страницыGlenn Beck SuicideLE PathwayОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (894)
- HE LE Vs CG Opposition Path 8Документ57 страницHE LE Vs CG Opposition Path 8LE PathwayОценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Life Extension Metabolism ControlДокумент66 страницLife Extension Metabolism ControlLE PathwayОценок пока нет
- Foliar FertilizerДокумент7 страницFoliar FertilizerDjugian GebhardОценок пока нет
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Management of Intra Abdominal Infections From A Global Perspective 2017 WSES Guidelines For Management of Intra Abdominal InfectionsДокумент34 страницыThe Management of Intra Abdominal Infections From A Global Perspective 2017 WSES Guidelines For Management of Intra Abdominal InfectionsJuan CastroОценок пока нет
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Modul Strategik Bab 1 Ting 5Документ6 страницModul Strategik Bab 1 Ting 5SK Pos TenauОценок пока нет
- NCM 103 RLE NOTESДокумент8 страницNCM 103 RLE NOTESgallardo.bettinarose.iОценок пока нет
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- MRCP 2 Nephrology NOTESДокумент74 страницыMRCP 2 Nephrology NOTESMuhammad HaneefОценок пока нет
- q3 Sci10 Unit1 Feedback MechanismsДокумент125 страницq3 Sci10 Unit1 Feedback MechanismsIvann EboraОценок пока нет
- L.D..Occlusion in FPDДокумент138 страницL.D..Occlusion in FPDApurva Deshmukh67% (3)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Vacuole FunctionДокумент8 страницVacuole Functionkbansal981Оценок пока нет
- Bfhi-Session-5-How Breastfeeding WorksДокумент16 страницBfhi-Session-5-How Breastfeeding Worksreema arshadОценок пока нет
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Traumatic Hyphema PDFДокумент10 страницTraumatic Hyphema PDFPratiwi SimanОценок пока нет
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Anxiety: What Are Some Symptoms of Anxiety?Документ3 страницыAnxiety: What Are Some Symptoms of Anxiety?Khairil AshrafОценок пока нет
- Aids 2013Документ404 страницыAids 2013kovaron80Оценок пока нет
- Science: Quarter 1 - Module 1Документ10 страницScience: Quarter 1 - Module 1RUTH PIANGОценок пока нет
- Nicotrol InhalerДокумент19 страницNicotrol InhalerdebysiskaОценок пока нет
- IlizarovДокумент62 страницыIlizarovAsh Moaveni100% (2)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Mobilization NotesДокумент239 страницMobilization NotesSuganya Balachandran100% (6)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- Hematology Analyzers, Coagulation Systems Product GuideДокумент4 страницыHematology Analyzers, Coagulation Systems Product GuideElvan Dwi WidyadiОценок пока нет
- Kotlyar 2016Документ1 страницаKotlyar 2016dasdsaОценок пока нет
- Super 108 DR LalДокумент8 страницSuper 108 DR Lalarkaprava paulОценок пока нет
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Radiation Biology: Presented By: Aarya.H.NairДокумент83 страницыRadiation Biology: Presented By: Aarya.H.NairAARYAОценок пока нет
- Neurodegenerative Cerebellar AtaxiaДокумент26 страницNeurodegenerative Cerebellar AtaxiaМилица МилошевићОценок пока нет
- Chapter 3: Movement of Substance Across The Plasma MembraneДокумент13 страницChapter 3: Movement of Substance Across The Plasma MembraneEma FatimahОценок пока нет
- Photosynthesis Question Booklet: Name . .Документ16 страницPhotosynthesis Question Booklet: Name . .ajithaОценок пока нет
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Anatomy and Physiology-REVIEWER-Practical ExamДокумент12 страницAnatomy and Physiology-REVIEWER-Practical ExamDeity Ann ReuterezОценок пока нет
- ENLSV3.0 ICP FinalДокумент7 страницENLSV3.0 ICP FinalGustavo HenrikeОценок пока нет
- ANAESTHESIOLOGYДокумент38 страницANAESTHESIOLOGYcollinsmagОценок пока нет
- NIOSH Investigation - CR 337 Fire LODDДокумент32 страницыNIOSH Investigation - CR 337 Fire LODDRamblingChiefОценок пока нет
- Ron Kurtz - The Body Reveals PDFДокумент161 страницаRon Kurtz - The Body Reveals PDFNemanja92% (12)
- Point MarmaДокумент2 страницыPoint MarmaAnonymous yzbnd8Оценок пока нет
- Long-Term Memory Encoding and RetrievalДокумент24 страницыLong-Term Memory Encoding and RetrievalThavasi mari selvam NОценок пока нет