Академический Документы
Профессиональный Документы
Культура Документы
A Competitiveness Policy For The Medical Technology Industry: Six Policy Proposals To Sustain American Leadership
Загружено:
AdvaMedLCIИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
A Competitiveness Policy For The Medical Technology Industry: Six Policy Proposals To Sustain American Leadership
Загружено:
AdvaMedLCIАвторское право:
Доступные форматы
A Competitiveness Policy for the Medical Technology Industry: Six Policy Proposals to Sustain American Leadership
1. Innovation in the life sciences must be a government priority. Since the ability of the life science industries to thrive is affected by a broad range of government policies across many agencies, it is critical that that supporting medical innovation be a priority for the whole government. A. An office of medical innovation policy should be created in the White House. This office would have oversight responsibility for major proposed and current government policies to assure that they support medical innovation. The office would serve as a focal point for groups and individuals advocating for medical innovation and could develop an innovation index to track how well the United States measures up to its major competitors in policies to encourage innovation. B. An innovation impact statement would be required for major regulations or other actions that affect the health sector. This statement would be analogous to an environmental impact statement. The goal would be to assure that every agency takes into account the effect of its actions on medical innovation and related domestic employment, and economic growth in promulgating government rules. 2. The FDA review process must be reformed. The FDA must set a goal of achieving a review and approval process that is as predictable, consistent, and timely as our European competitors, while continuing to assure that products are safe and effective. A. FDA must reduce total review times, not just time on the FDA clock, to a level that will significantly speed up review of both 510(k) and PMA products, including reforming the de novo process to make it an efficient and workable system for class II products with no predicate. B. FDA must effectively implement least burdensome processes throughout its operations to eliminate requirements that are not necessary to protect public health. C. FDA must streamline the IDE process to assure timely initiation of clinical trials.
D. FDA must develop a full range of guidance documents that identify FDAs requirements for a specific product submission to ensure a timely and consistent review process. E. FDA must adopt the risk-based review pathway for diagnostic tests. F. The FDA must take steps to ensure that its staff is properly trained, has access to independent scientific and technological information, and to develop a program to monitor the predictability and consistency of the review process. G. FDA must take steps to converge its regulatory practices with the principles established by the Global Harmonization Task Force. 3. Payment policy must support medical innovation. Medicare, Medicaid, and private insurers alike must assure that the new payment modalities established by health reform to provide incentives for quality and cost control also support medical progress, innovation and access to appropriate technology. The current Medicare coding and payment processes must be improved to allow more rapid recognition of new technologies. A. New payments systems such as accountable care organizations, bundling, and value-based purchasing should include specific provisions to avoid penalizing health care organizations or individual providers for offering patients the opportunity to benefit from new treatments that are not yet the standard of care. B. New payment systems should be carefully designed to support continued patient access to care appropriate for their individual needs and to recognize the long-term value of treatments. C. CMS should reform the process of coding and determining appropriate payment to avoid delays of up to two years or more before a treatment can be properly recognized for payment purposes. D. CMS should reform payment for new diagnostic tests to encourage the development of high value diagnostics and of personalized medicine. 4. A vigorous trade policy must support export growth and provide a level playing field for U.S.-based manufacturing. If trade barriers remain or increase, U.S. efforts to improve domestic competitiveness and expand exports would be undermined. Companies will relocate outside the U.S. to manufacture behind the barriers and foreign companies will thrive at the expense of U.S. competitors. Other countries are pursuing bilateral and regional trade agreements that will put U.S. manufacturers at a competitive disadvantage. Countries in the developing world are increasingly using regulatory policy to promote domestic industries or to force U.S. companies to locate research, development, and manufacturing
within their borders. Small and medium size companies need additional assistance to become successful exporters. A. The Presidents National Export Initiative (NEI) should make bilateral and regional free trade agreements (and associated medical technology sectoral agreements) with developed and developing markets alike a priority, including ratification of the Korean-US free trade agreement, negotiation of the TransPacific Partnership free trade agreement and expanding the agreement to include additional Asia-Pacific countries, including Japan. B. The Administration should continue its policy of vigorous opposition to non-tariff barriers to trade, especially use of regulatory policy to set up artificial barriers to imported products and to force local location of research and development and manufacturing by multinational firms. The Administration should support existing and new trade forums that allow government officials and industry representatives to work together to identify and address barriers to trade. FDA should be part of the team working with trade authorities and indicate that assistance to foreign firms seeking to meet U.S. regulatory requirements is conditional on fair treatment of U.S. firms by foreign regulatory authorities. C. The Administration should make regulatory harmonization by developing countries a trade priority, including achieving a commitment next year to regulatory harmonization by 2020 at the Leaders meeting of the Asia Pacific Economic Cooperation forum, based on the principles adopted by the Global Harmonization Task Force. D. Small and medium size enterprises represent the lifeblood of medical technology innovation. Exporting to foreign markets is particularly difficult for companies with little or no foreign trade experience. Under the NEI, US Government agencies including USTR, SBA, and Commerce should vigorously pursue policies to assist small and medium size companies to overcome their lack of experience and specialized knowledge, and other obstacles to competing in export markets. 5. Strategic tax policies to level the playing field must be implemented. American tax policy must support research and development (R&D) intensive industries at a level sufficient to level the playing field with foreign governments eager to attract American jobs and develop home-grown competitors to American firms. The R&D tax credit must be reformed and made more generous; tax incentives need to created for keeping R&D based manufacturing in America. The medical device excise tax should be repealed. A. The Research and Development Tax Credit needs to be made permanent; the level of the credit needs to be raised so that it is as
good or better than the credits provided by our major competitors; the administration of the credit should be substantially simplified; the credit should support investment in building research infrastructure, including construction of facilities and purchase of equipment; and the tax code should provide incentives equivalent to the credit for companies with no profits, so that small and startup companies, which create a disproportionate share of breakthrough treatments, can receive benefits at the time of greatest need. B. Manufacturing based on R&D wholly or predominantly conducted in the United States should be eligible for a lower corporate tax rate to reduce the cost advantage that research and development intensive companies locating manufacturing abroad enjoy in the form of lower general corporate taxes, special tax breaks, and direct subsidies. C. The medical device excise tax should be repealed, since it absorbs resources that could otherwise be used for research and development or employment expansion and disproportionately burdens American firms vis--vis foreign competitors. D. The United States should move towards a corporate tax system that provides greater parity with our major competitors in tax rates and treatment of foreign earnings. 6. The American research and development infrastructure must be sustained and improved. American policy must support the maintenance and growth of an R&D infrastructure second to none, with special emphasis on creating the structures necessary to support translational R&D directed at commercialization. A. America must maintain and expand its commitment to basic research and to graduate research and training programs through the NIH and NSF. B. Research programs that support moving research farther along the development spectrum toward actual treatments and that support start-up companies developing breakthrough treatments should be improved and expanded, including increasing funding, eligibility, and maximum grant size for the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR programs) and fully funding the Cures Acceleration Network. Additionally, the federal government should provide grant funding to states and localities seeking to establish or expand bioscience research and development clusters. C. Programs should be established to more effectively tap the vast intellectual resources of our nations universities and academic health centers, including creating NIH funded Industry-University Cooperative Research Centers analogous to a long-standing and
successful program at the NSF and providing federal technical assistance to establish best practices and improve the effectiveness of university technology transfer programs. D. Institutional Review Board activities should be streamlined to reduce barriers to initiating collection of clinical data on new products, particularly for multicenter trials, without sacrificing protection of human subjects.
Вам также может понравиться
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Marzano's Compendium: Acknowledging Adherence To Rules and ProceduresДокумент28 страницMarzano's Compendium: Acknowledging Adherence To Rules and ProceduresClaudia VelascoОценок пока нет
- Math - MAA Problem Series List PDFДокумент2 страницыMath - MAA Problem Series List PDFadasdОценок пока нет
- STRUCTURAL - Chapter 14 - Explicit Dynamics Analysis (UP19980818)Документ26 страницSTRUCTURAL - Chapter 14 - Explicit Dynamics Analysis (UP19980818)Rory Cristian Cordero RojoОценок пока нет
- Managing The Multigenerational Workplace White PaperДокумент19 страницManaging The Multigenerational Workplace White PaperSanghamitra DanОценок пока нет
- MATH 3rd Grading Summative Test 4Документ3 страницыMATH 3rd Grading Summative Test 4Aubrey Gay SarabosquezОценок пока нет
- The Value of Medical Technology: Impact of DiabetesДокумент2 страницыThe Value of Medical Technology: Impact of DiabetesAdvaMedLCIОценок пока нет
- Infographic: IMPACT OF HEART DISEASEДокумент1 страницаInfographic: IMPACT OF HEART DISEASEAdvaMedLCIОценок пока нет
- The Value of Medical Technology: Managing Chronic PainДокумент2 страницыThe Value of Medical Technology: Managing Chronic PainAdvaMedLCIОценок пока нет
- The Importance of Diagnostic Tests in Fighting Infectious DiseasesДокумент2 страницыThe Importance of Diagnostic Tests in Fighting Infectious DiseasesAdvaMedLCIОценок пока нет
- Infographic: IMPACT OF MUSCULOSKELETAL DISEASEДокумент1 страницаInfographic: IMPACT OF MUSCULOSKELETAL DISEASEAdvaMedLCIОценок пока нет
- Infographic: HEALTHY SAVINGS: MEDICAL TECHNOLOGY & THE ECONOMIC BURDEN OF DISEASEДокумент1 страницаInfographic: HEALTHY SAVINGS: MEDICAL TECHNOLOGY & THE ECONOMIC BURDEN OF DISEASEAdvaMedLCIОценок пока нет
- Chronic Pain Management Fact SheetДокумент2 страницыChronic Pain Management Fact SheetAdvaMedLCIОценок пока нет
- Infographic: IMPACT OF DIABETESДокумент1 страницаInfographic: IMPACT OF DIABETESAdvaMedLCIОценок пока нет
- LCI Wound Treatment Toolkit 2015Документ8 страницLCI Wound Treatment Toolkit 2015AdvaMedLCIОценок пока нет
- Wound Treatment Infographic 2015Документ1 страницаWound Treatment Infographic 2015AdvaMedLCIОценок пока нет
- Infographic: HEALTHY SAVINGS: MEDICAL TECHNOLOGY & THE ECONOMIC BURDEN OF DISEASEДокумент1 страницаInfographic: HEALTHY SAVINGS: MEDICAL TECHNOLOGY & THE ECONOMIC BURDEN OF DISEASEAdvaMedLCIОценок пока нет
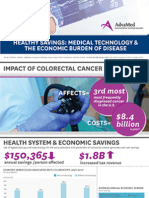
- Infographic: IMPACT OF COLORECTAL CANCERДокумент1 страницаInfographic: IMPACT OF COLORECTAL CANCERAdvaMedLCIОценок пока нет
- Innovation Agenda: February 2015Документ4 страницыInnovation Agenda: February 2015AdvaMedLCIОценок пока нет
- 2015 Innovation AgendaДокумент1 страница2015 Innovation AgendaAdvaMedLCIОценок пока нет
- MedTech Prices Lag Behind CPI, Other Medical GoodsДокумент2 страницыMedTech Prices Lag Behind CPI, Other Medical GoodsAdvaMedLCIОценок пока нет
- Pulse Redefining Medical Technology InnovationДокумент64 страницыPulse Redefining Medical Technology InnovationAdvaMedLCIОценок пока нет
- Estimates of Medical Device Spending in The United StatesДокумент17 страницEstimates of Medical Device Spending in The United StatesAdvaMedLCIОценок пока нет
- Impact of The Medical Device Excise TaxДокумент5 страницImpact of The Medical Device Excise TaxAdvaMedLCIОценок пока нет
- The Anatomy of Medical ResearchДокумент16 страницThe Anatomy of Medical ResearchAdvaMedLCI0% (1)
- Social Media Stroke ToolkitДокумент4 страницыSocial Media Stroke ToolkitAdvaMedLCIОценок пока нет
- AdvaMed Device Tax ReportДокумент5 страницAdvaMed Device Tax ReportAdvaMedLCIОценок пока нет
- Diabetes Infographic: The Value of Medical Technology: Controlling & Treating DiabetesДокумент1 страницаDiabetes Infographic: The Value of Medical Technology: Controlling & Treating DiabetesAdvaMedLCIОценок пока нет
- The Value of Medical Technology in Controlling and Treating DiabetesДокумент8 страницThe Value of Medical Technology in Controlling and Treating DiabetesAdvaMedLCIОценок пока нет
- Recent Average Price Trends For Implantable Medical Devices, 2007-2011Документ7 страницRecent Average Price Trends For Implantable Medical Devices, 2007-2011AdvaMedLCIОценок пока нет
- Pulse Medical Technology Report 2012Документ52 страницыPulse Medical Technology Report 2012AdvaMedLCIОценок пока нет
- The Value of Joint Replacement in Treating Patients With OsteoarthritisДокумент6 страницThe Value of Joint Replacement in Treating Patients With OsteoarthritisAdvaMedLCIОценок пока нет
- Oct 2012 King Report FINALДокумент16 страницOct 2012 King Report FINALAdvaMedLCIОценок пока нет
- Sustaining American Leadership: A Competitiveness Policy For The Medical Technology IndustryДокумент3 страницыSustaining American Leadership: A Competitiveness Policy For The Medical Technology IndustryAdvaMedLCIОценок пока нет
- Employment Effects of The New Excise Tax On The Medical Device IndustryДокумент30 страницEmployment Effects of The New Excise Tax On The Medical Device IndustryAdvaMedLCIОценок пока нет
- MS - BDA Lec - Recommendation Systems IДокумент31 страницаMS - BDA Lec - Recommendation Systems IJasura HimeОценок пока нет
- Analysis of Portal Frame Structure With ETABS PDFДокумент19 страницAnalysis of Portal Frame Structure With ETABS PDFAnonymous OynOOfОценок пока нет
- Final Assignment Naronda WrightДокумент2 страницыFinal Assignment Naronda Wrightapi-290301506Оценок пока нет
- Job Description Mechanical Design EngineersДокумент1 страницаJob Description Mechanical Design Engineersyinkaakins2001Оценок пока нет
- Dukungan Suami Terhadap Pemberian Asi Eksklusif PaДокумент11 страницDukungan Suami Terhadap Pemberian Asi Eksklusif PayolandaОценок пока нет
- Facebook Romanian Translation Style GuideДокумент20 страницFacebook Romanian Translation Style GuideLeonОценок пока нет
- Critical Issues in JournalismДокумент5 страницCritical Issues in JournalismChad McMillenОценок пока нет
- Science Investigatory Project PDFДокумент2 страницыScience Investigatory Project PDFNick john CaminadeОценок пока нет
- Schnugh V The State (Bail Appeal) Case No 92-09 (Van Niekerk & Parker, JJ) 31jan'11 PDFДокумент25 страницSchnugh V The State (Bail Appeal) Case No 92-09 (Van Niekerk & Parker, JJ) 31jan'11 PDFAndré Le RouxОценок пока нет
- July 1Документ5 страницJuly 1June RañolaОценок пока нет
- A Disease, Disorder, Illness or Condition: How To Label Epilepsy?Документ5 страницA Disease, Disorder, Illness or Condition: How To Label Epilepsy?HaniОценок пока нет
- Adu-Yeboah Emmanuel. Mphil in Leadership, UpsaДокумент146 страницAdu-Yeboah Emmanuel. Mphil in Leadership, UpsaEmmanuel Adu-YeboahОценок пока нет
- (1997) Process Capability Analysis For Non-Normal Relay Test DataДокумент8 страниц(1997) Process Capability Analysis For Non-Normal Relay Test DataNELSONHUGOОценок пока нет
- NCI Operations Manual June 28Документ225 страницNCI Operations Manual June 28bile_driven_opus100% (1)
- Exam 1Документ61 страницаExam 1Sara M. DheyabОценок пока нет
- Gold Exp C1 U4 Lang Test BДокумент2 страницыGold Exp C1 U4 Lang Test Bmaituti1Оценок пока нет
- Mayo Medical School: College of MedicineДокумент28 страницMayo Medical School: College of MedicineDragomir IsabellaОценок пока нет
- PT326-Round2 Expt3 Batch19Документ6 страницPT326-Round2 Expt3 Batch19Radhey MeenaОценок пока нет
- Advances Chemical Engineering PDFДокумент248 страницAdvances Chemical Engineering PDFDaiane SantanaОценок пока нет
- Team Meeting - 6th MayДокумент11 страницTeam Meeting - 6th MaySachin SharmaОценок пока нет
- Mini Fellowship Program OutlineДокумент4 страницыMini Fellowship Program OutlineVijayraj GohilОценок пока нет
- MBTI StepДокумент21 страницаMBTI StepRedgie G. GabaneОценок пока нет
- MODULI I LITERATURA Za ALAN Master Optometriju - OdtДокумент3 страницыMODULI I LITERATURA Za ALAN Master Optometriju - OdtVladislav StevanovicОценок пока нет
- Sir Josiah Stamp, The Science of Social AdjustmentДокумент191 страницаSir Josiah Stamp, The Science of Social Adjustmentmaivin2Оценок пока нет
- The Use of Electrical Resistivity Tomography (ERT) To Delineate W PDFДокумент76 страницThe Use of Electrical Resistivity Tomography (ERT) To Delineate W PDFConstantin UngureanuОценок пока нет