Академический Документы
Профессиональный Документы
Культура Документы
The Adrenal Glands
Загружено:
ashgee1Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
The Adrenal Glands
Загружено:
ashgee1Авторское право:
Доступные форматы
CHAPTER
39
The Adrenal Glands
Quan-Yang Duh, MD
and
Michael W. Yeh, MD
History Anatomy and Embryology Normal Histopathology Biochemistry and Physiology Inborn Errors of Metabolism: Congenital Adrenal Hyperplasia Adrenal Insufciency Diseases of the Adrenal Cortex Diseases of the Adrenal Medulla The Incidentally Discovered Adrenal Mass (Incidentaloma) Metastases to the Adrenal Gland Technical Aspects of Adrenalectomy
Medicine for their groundbreaking work on the adrenocortical hormones. The Austrian-born endocrinologist Hans Selye rst described the stress response in mammals in 1936 and made major contributions to understanding of the hypothalamic-pituitary-adrenal (HPA) axis. Roger Guillemin, Andrew Schally, and Rosalyn Yalow were awarded the Nobel Prize in 1977 for characterizing the peptide hormones of the brain that underlie the HPA axis as we now understand it.1,2
ANATOMY AND EMBRYOLOGY General and Developmental Aspects
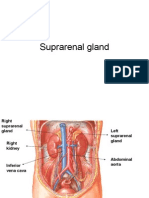
The adrenal glands are paired, mustard-colored structures that are positioned superior and slightly medial to the kidneys in the retroperitoneal space (Fig. 39-1). They are attened and roughly pyramidal (right) or crescent shaped (left) and weigh approximately 4 g each. The adrenals are among the most highly perfused organs in the body, with blood ow of 2000 mL/kg/min, behind only the kidney and the thyroid. In most respects the cortex and medulla can be considered two completely distinct organs that happen to colocalize during development. The two portions have disparate embryologic origins. The primordial cortex arises from the coelomic mesodermal tissue near the cephalic end of the mesonephros during the fourth to fth week of gestation. Biosynthetic activity can be detected as early as the seventh week. The cortical cell mass dominates the fetal adrenal at 4 months of development, and steroidogenesis reaches its maximum during the third trimester. The adrenal medulla arises from ectodermal tissues of the embryonic neural crest. It develops in parallel with the sympathetic nervous system, beginning in the fth to sixth week of gestation. From their original position adjacent to the neural tube, neural crest cells migrate ventrally to assume a para-aortic position near the developing adrenal cortex. There, they
HISTORY
The adrenal glands were rst described by the Italian anatomist Bartolomeo Eustachi in 1563. The German comparative anatomist Albert von Klliker (1817-1905), who noted the presence of adrenals in a number of vertebrate species, is credited with rst identifying two distinct portions of the adrenal gland, namely, the cortex and medulla. Although Thomas Addison described the clinical features of primary adrenal failure in 1855, it was not until nearly a century later that the adrenal hormones were fully isolated and characterized. Adrenaline (or epinephrine) was rst isolated from adrenal extract at the turn of the century. Steroid hormones were crystallized from cortical extract (cortin) by Swiss and American investigators in the 1930s, but their highly similar chemical structures made isolation of the individual compounds challenging. Edward Kendall, Tadeus Reichstein, and Philip Hench jointly received the 1950 Nobel Prize in Physiology or
997
998
Section VIII Endocrine
Right and left inferior phrenic arteries Inferior vena cava
Right adrenal gland Right adrenal vein
Left adrenal gland
Left inferior phrenic vein
Left inferior adrenal artery
Right renal artery and vein
Left adrenal vein
Left kidney
Left renal artery and vein
Inferior vena cava
Abdominal aorta
Figure 39-1 Anatomy of the adrenal glands. A, Left and right adrenal glands in situ.
differentiate into the chromafn cells that make up the adrenal medulla.3 This course of embryologic development yields certain surgically relevant sequelae. Both cortical and medullary tissue can be found at extra-adrenal sites (Fig. 39-2). The range of potential sites is wider for chromafn tissue than for cortical tissue, presumably because of the longer path of migration for the former. Pheochromocytomas may arise in extra-adrenal sites more commonly than previously thought (see later). When extra-adrenal, pheochromocytomas are also called paragangliomas.
by its proper capsule, in addition to sharing Gerotas fascia with the kidneys. The adrenal capsules are immediately associated with the perirenal fat.
Vasculature
Knowledge of the macroscopic vascular anatomy of the adrenal glands is essential for proper surgical management. It is important to conceptualize that although the arterial supply is diffuse, the venous drainage of each gland is usually solitary. The arterial supply arises from three distinct vessels: the superior adrenal arteries from the inferior phrenic arteries, the small middle adrenal arteries from the juxtaceliac aorta, and the inferior adrenal arteries from the renal arteries. Of these, the inferior is the most prominent and is commonly a single identiable vessel. The left adrenal vein is approximately 2 cm long and drains into the left renal vein after joining the inferior phrenic vein. The right adrenal vein is typically as short as it is wide (0.5 cm) and drains directly into the vena cava. This conguration presents a surgical challenge that will be revisited in the technical section of this chapter. In up to 20% of individuals, the right adrenal vein may drain into an accessory right hepatic vein or into the vena
Relationships
The right adrenal gland abuts the posterolateral surface of the retrohepatic vena cava. The right adrenal fossa is bounded by the right kidney inferolaterally, the diaphragm posteriorly, and the bare area of the liver anterosuperiorly. The left adrenal gland lies between the left kidney and aorta, with its inferior limb extending farther caudad toward the renal hilum than the right adrenal. The other relationships of the left adrenal gland are the diaphragm posteriorly and the tail of the pancreas and splenic hilum anteriorly. Each adrenal gland is enveloped
Chapter 39 The Adrenal Glands
999
Stomach
Spleen Adrenal gland
Pancreas Liver
Adrenal gland Kidney
Inferior vena cava Duodenum Kidney
Pancreas Stomach Liver
Inferior vena cava Spleen Adrenal gland Kidney Diaphragm Diaphragm Adrenal gland
C
Figure 39-1, contd B, Relationships of the left adrenal gland. C, Relationships of the right adrenal gland.
Adrenal T1 Medullary Cortical
cava at or near the conuence of such a vein.4 Vigilance toward this variant and others (Fig. 39-3) may reduce the likelihood of intraoperative venous hemorrhage during right adrenalectomy.
NORMAL HISTOPATHOLOGY
The cortex is approximately 2 mm thick and accounts for greater than 80% of the mass of the gland. It is made up of three layers (Fig. 39-4). The outer zona glomerulosa is a thin layer of relatively small cells with moderately eosinophilic, lipid-poor cytoplasm. It has an undulating inner border and normally does not form a complete circumferential layer. The majority of the adrenal cortex is formed by the zona fasciculata, a middle layer composed of long radial columns of large, clear, lipid-laden cells. The inner zona reticularis is made up of small nests of compact, eosinophilic cells. The adrenal medulla con-
Figure 39-2 Sites of extra-adrenal cortical and medullary tissue.
1000
Section VIII Endocrine
Right hepatic vein Adrenal
Middle and left hepatic veins
Right hepatic vein Adrenal
Middle and left hepatic veins
Territory of potential venous confluence (multiple veins may be present)
Adrenal vein
Kidney
Kidney
Renal Inferior vein vena cava Territory
Renal Inferior vein vena cava Normal (single vein directly into IVC) Middle and left hepatic veins
A
Middle and left hepatic veins
Right hepatic vein Adrenal
Right hepatic vein Adrenal
Adrenal vein Kidney Kidney
Adrenal vein
Inferior Renal vein vena cava IVC/renal vein trifurcation
Inferior Renal vein vena cava Renal vein confluence
D
Figure 39-3 Variations in right adrenal vein anatomy. A, Territory of potential right adrenal vein conuence.
B, Normal (>80%)single vein directly into the inferior vena cava (IVC). C, IVC/renal vein trifurcation. D, Renal vein conuence.
sists of clusters and short cords of chromafn cells, which are large, polyhedral, and packed with basophilic secretory granules. Catecholamines within these granules yield a brown-colored reaction when treated with chromium salts, thus giving the cells their name. In contrast to the cortex, the adrenal medulla is richly endowed with autonomic nerve bers and ganglion cells. Sympathetic bers
synapse directly with the chromafn cells and constitute an interface between the nervous and endocrine systems.5 The microvasculature of the adrenal gland functionally unies the cortex and medulla. The adrenal arteries arborize extensively before entering the capsule to form a subcapsular plexus. Blood ows centripetally through
Chapter 39 The Adrenal Glands
1001
Right hepatic vein Adrenal
Middle and left hepatic veins
Right hepatic vein Adrenal
Middle and left hepatic veins
Adrenal vein
Adrenal vein
Kidney
Kidney
Renal Inferior vein vena cava High single vein
Renal Inferior vein vena cava IVC/right hepatic vein trifurcation Middle and left hepatic veins
E
Right hepatic vein Adrenal
Adrenal vein
Kidney
Inferior Renal vein vena cava Right hepatic vein confluence
G
Figure 39-3, contd E, High single vein into the IVC. F, IVC/right hepatic vein trifurcation. G, Right hepatic vein
conuence.
capillaries in the zona glomerulosa and zona fasciculata before forming a deep plexus within the zona reticularis. From there, steroid-enriched postcapillary blood enters the medulla, where cortisol drives expression of phenylethanolamine-N-methyltransferase (PNMT). PNMT is responsible for conversion of norepinephrine to epinephrine. This microvascular arrangement is essentially a portal system between the cortex and the medulla.
BIOCHEMISTRY AND PHYSIOLOGY Adrenal Steroid Biosynthesis
Adrenal steroid biosynthesis begins with transport of cholesterol to the inner mitochondrial membrane by the steroidogenic acute regulatory protein (StAR) (Fig. 39-5).6 Cholesterol then undergoes a series of oxidative reac-
1002
Section VIII Endocrine
latter being active in humans. Aldosterone is generated by oxidation of corticosterone at the carbon 18 position by CYP11B2 localized to the zona glomerulosa. CYP17 expression is conned to the zona fasciculata and zona reticularis, thus accounting for synthesis of glucocorticoids and adrenal sex steroids in these regions.
Steroid Hormone Physiology and Metabolism
Steroid hormones belong to a general class of lowmolecular-weight, lipophilic signaling molecules that act by entering cells and binding to intracellular receptors. This group of hormones also includes thyroid hormone, retinoids, and vitamin D. Hormone binding results in alterations in gene expression that show a delayed and prolonged response when compared with the changes induced by peptide hormones, which act by binding to cell surface receptors. In the circulation, endogenous steroid hormones are largely bound to highly specic binding globulins. Serum levels of these proteins (and hence free hormone levels) can be altered by certain physiologic and disease states such as pregnancy, nephrotic syndrome, and cirrhosis. Metabolism of both endogenous and pharmacologic steroids generally proceeds via hydroxylation, sulfonation, or conjugation (or any combination of these processes) to glucuronic acid in the liver, followed by urinary excretion. The regulation and physiologic actions of individual steroid hormones are discussed in the following sections.
Capsule
Glomerulosa Faciculata
Glucocorticoids
Reticularis
B
Figure 39-4 Normal adrenal histopathology. A, Low-power view showing the adrenal cortex (C) and medulla (M). B, Mediumpower view demonstrating individual layers of the adrenal cortex. The thickness of the zona glomerulosa varies along its length. (Photomicrographs courtesy of Anthony Gill, MD).
tions catalyzed predominantly by membrane-associated enzymes belonging to the cytochrome P-450 (CYP) family. Cleavage of the cholesterol side chain yields the hormonally inactive compound pregnenolone, the immediate precursor to adrenal steroid hormones. Serial oxidation by CYP17 (moving rightward on the chart) converts pregnenolone and progesterone into the major adrenal sex steroids dehydroepiandrosterone (DHEA) and androstenedione. Additional enzymatic steps conned to the gonads (not shown) generate testosterone, estrone, and estradiol from androstenedione. Oxidation of pregnenolone and 17-hydroxypregnenolone at the carbon 3 position by 3-hydroxysteroid dehydrogenase, followed by the action of CYP21A2 and CYP11B1 (moving downward on the diagram), yields the major mammalian glucocorticoids corticosterone and cortisol, with only the
Release of corticotropin-releasing factor (CRF) into the hypothalamic-pituitary portal system by hypothalamic neurons results in secretion of adrenocorticotropic hormone (ACTH) by the anterior pituitary. Synthesis of pro-opiomelanocortin (POMC), the large precursor peptide to ACTH, is also up-regulated. ACTH binds to a G proteincoupled receptor on the adrenocortical cell surface and stimulates glucocorticoid secretion, among other effects. Steroidogenesis is acutely up-regulated via increased StAR-mediated cholesterol transport and pregnenolone synthesis by CYP11A1 (cholesterol side chain cleavage enzyme). Chronically, ACTH increases transcription of all steroidogenic enzymes and supports maintenance of normal adrenal cell mass. ACTH is released in a pulsatile fashion that normally displays a circadian rhythm. The highest levels of ACTH, and thus cortisol, are generally detected on waking, with levels gradually declining through the day to reach a nadir in the early evening. This pattern must be considered when evaluating patients for glucocorticoid deciency or excess. Negative feedback by glucocorticoids occurs at both the hypothalamic and pituitary levels. Glucocorticoid hormones have broad-ranging effects on almost all organ systems in the body. As a rule, they generate a catabolic state that characterizes the bodys response to stress. The hormones are so named because they cause alterations in carbohydrate, protein, and lipid metabolism that have the net effect of increasing blood
StAR
Cholesterol transport to inner mitochondrial membrane
19 1 2 3 10 4 5 6 11 9 8 7 12
21 18 13 14 20 17
16 15
HO
Cholesterol carbon numbering Reactions confined to zonae fasciculata and reticularis
CH3 C=O CH3 C=O OH O
Cholesterol CYP11A1 Side chain cleavage
CYP17
CYP17
HO
HO
HO
Pregnenolone 3HSD
CH3 C=O
17-hydroxy pregnenolone 3HSD
CH3 C=O
Dehydroepiandrosterone (DHEA) 3HSD
O
CYP17
OH
CYP17
Progesterone CYP21A2
CH2OH C=O
17-hydroxy progesterone CYP21A2
CH2OH C=O OH
Androstenedione
Deoxycorticosterone CYP11B1
CH2OH C=O HO
11-deoxycortisol CYP11B1
CH2OH C=O HO OH
Corticosterone CYP11B2
CH2OH C=O HO
Cortisol
Aldosterone Reaction confined to zona glomerulosa
Figure 39-5 Adrenal steroid biosynthesis. Reactions conned to the zone glomerulosa are shaded turquoise; those
conned to the zonae fasciculata and reticularis are shaded orange. Human mineralocorticoids are indicated in yellow, glucocorticoids in green, sex steroids in blue.
1004
Section VIII Endocrine
glucose concentrations. Hepatic glucose output is elevated by up-regulation of gluconeogenesis, and net glycogen deposition occurs. Glucose uptake by peripheral tissues is directly inhibited. Glucocorticoids stimulate lipolysis with the release of free fatty acids into the circulation, and a general state of insulin resistance is induced that results in protein catabolism. Fatty acids and amino acids serve as energy sources and substrate for gluconeogenesis. In the cardiovascular system, glucocorticoids exert a permissive and enhancing effect on catecholamine signaling by sensitizing arterial smooth muscle cells to -adrenergic stimulation and increasing catecholamine concentrations in neuromuscular junctions.7 Cardiac contractility and peripheral vascular tone are thus maintained, which explains why the hemodynamic collapse that accompanies acute adrenal insufciency can be remedied by glucocorticoid administration. Glucocorticoids are potent anti-inammatory and immunosuppressive agents that act at many levels. Acutely, glucocorticoids reduce circulating lymphocyte and eosinophil counts while increasing neutrophil counts. Lymphocyte apoptosis is promoted, cytokine and immunoglobulin production is decreased, and histamine release is suppressed. Glucocorticoids also reduce prostaglandin synthesis via inhibition of phospholipase A2. Additional pathologic effects of glucocorticoids are discussed later in the section on glucocorticoid excess.
synthesized in both the adrenal and liver) is regulated by ACTH and other incompletely understood mechanisms. Of the three, androstenedione is produced in the smallest quantities. The physiologic effects of adrenal sex steroids are generally weak in comparison to the gonadal sex steroids, particularly in males. In females, peripheral conversion of DHEA and DHEA-S to more potent androgens, including androstenedione, testosterone, and dihydrotestosterone, supports normal pubic and axillary hair growth and may play a role in maintaining libido and a sense of well-being.
Catecholamine Biosynthesis and Physiology
Synthesis of catecholamines in the adrenal medulla begins with the hydroxylation of tyrosine, a rate-limiting step that generates dihydroxyphenylalanine (L-dopa) in the cytosol (Fig. 39-6). Decarboxylation of L-dopa generates dopamine, which is taken up by neurosecretory granules and -hydroxylated to form norepinephrine. Epinephrine is created by the action of PNMT, which unlike the other enzymes involved in catecholamine synthesis, is localized to the chromafn cells of the adrenal medulla and organ of Zuckerkandl. Sympathetic stimulation of the adrenal medulla results in depolarization of the chromafn cell membrane and release of stored catecholamines into the circulation. Basal levels of adrenal catecholamine secretion are normally low, although large (up to 50-fold) increases in levels may be observed in response to major physiologic or psychological stressors. Target tissue responses are mediated by - and -adrenergic receptors. -Adrenergic receptors display greater afnity for norepinephrine than for epinephrine, and the opposite is true for -adrenergic receptors. Stimulation of 1-receptors in the myocardium results in an increase in heart rate and contractility. Stimulation of 2-receptors results in smooth muscle relaxation in tissues such as the uterus, bronchi, and skeletal muscle arterioles. 1-Receptors mediate vasoconstriction in tissues such as the skin and gastrointestinal tract. 2-Receptors exist in presynaptic locations in the central nervous system, where they mediate attenuation of sympathetic outow. The net effect of adrenal catecholamine release is to augment blood ow and delivery of oxygen to the brain, heart, and skeletal muscle (which are essential for the ght or ight response) at the expense of other organ systems.
Mineralocorticoids
Release of aldosterone from the zona glomerulosa is principally regulated by angiotensin II and the blood potassium level. The renin-angiotensin-aldosterone axis is responsive to delivery of sodium to the distal convoluted tubule of the kidney. Low sodium delivery, which occurs in states such as hypovolemia, shock, renal artery vasoconstriction, and hyponatremia, stimulates the release of renin from the juxtaglomerular apparatus. The prohormone angiotensinogen is synthesized by the liver and is cleaved to inactive angiotensin I by renin. Further cleavage of angiotensin I by angiotensin-converting enzyme in the lungs and elsewhere yields angiotensin II, a potent vasoconstrictor and stimulator of aldosterone release. Aldosterone release is also highly sensitive to minute changes in the blood potassium level. Hypokalemia reduces aldosterone release by suppressing renin secretion and also by acting directly at the zona glomerulosa. Hyperkalemia has the opposite effect. Aldosterone regulates circulating uid volume and electrolyte balance by promoting sodium and chloride retention in the distal tubule. Potassium and hydrogen ions are secreted into urine. Acutely, expansion of extracellular uid volume and a rise in blood pressure are observed after aldosterone infusion. Negative feedback occurs primarily via an increase in sodium delivery to the distal tubule, which suppresses release of renin.
Catecholamine Clearance
Catecholamines are potent, short-acting compounds with a plasma half-life on the order of 1 minute. Their presence in synapses and the circulation is controlled by tight negative regulation via both reuptake and degradation. Degradation pathways merit some discussion because they generate the metabolites commonly measured in the biochemical evaluation of pheochromocytoma (discussed later). Epinephrine and norepinephrine are inactivated by one or both of the following enzymes: monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT) (see Fig. 39-6). Initial methylation by COMT yields meta-
Adrenal Sex Steroids
Secretion of the adrenal androgens androstenedione, DHEA, and DHEA-S (the sulfonated derivative of DHEA,
Chapter 39 The Adrenal Glands
1005
SYNTHESIS
HO CH2
COOH CH NH2
Tyrosine Tyrosine hydroxylase
HO COOH HO CH2 CH NH2
Dihydroxyphenylalinine (L-dopa) DOPA decarboxylase
HO
HO
CH2
CH2
NH2
Dopamine Dopamine -hydroxylase
HO OH HO CH CH2 NH2
Phenylethanolamine N-methyltransferase
HO
HO OH CH CH2 NH CH3
Norepinephrine Catechol-O-methyl transferase Monoamine oxidase
HO OH HO CH CH2 NH2 HO OH CH O C OH
Epinephrine Monoamine oxidase Catechol-O-methyl transferase
CH3O
CH3O OH HO CH CH2 NH CH3
Normetanephrine
3,4-Dihydroxymandelic acid Catechol-O-methyl transferase
CH3O OH HO CH O C OH
Metanephrine
Monoamine oxidase
Monoamine oxidase
Vanillylmandelic acid (VMA) DEGRADATION
Figure 39-6 Catecholamine biosynthesis and metabolism. Synthetic steps are shaded orange; degradative steps are shaded turquoise. Major catecholamines are indicated in green, major metabolites in yellow.
nephrine and normetanephrine, which can be detected in both plasma and urine. Their relatively stable plasma levels, which contrast with the high-amplitude uctuations seen in plasma epinephrine and norepinephrine levels, make them attractive diagnostic markers.8 The sequential action of MAO and COMT generates the major nal product vanillylmandelic acid. Catecholamine metabolites are excreted in urine, sometimes after sulfonation or conjugation to glucuronic acid in the liver.
INBORN ERRORS OF METABOLISM: CONGENITAL ADRENAL HYPERPLASIA
Congenital adrenal hyperplasia (CAH) is a relatively common inherited disorder that provides insight into mechanisms of steroid biosynthesis and negative feedback. Although six enzyme defects are known to cause CAH, more than 90% are caused by CYP21A2 deciency
1006
Section VIII Endocrine
Table 39-1
Properties of Endogenous and Commonly Used Pharmacologic Glucocorticoids
IV/PO* COMMON TRADE NAME RELATIVE POTENCY DAILY PHYSIOLOGIC DOSE DOSING INTERVAL
COMPOUND
Cortisol = hydrocortisone Cortisone Prednisone Prednisolone Methylprednisolone Dexamethasone
Both PO PO PO Both Both
Cortef (PO) Solu-Cortef (IV) Medrol (PO) Solu-Medrol (IV) Decadron
1 0.8 4 4 5 25
20 mg 25 mg 5 mg 5 mg 4 mg 1 mg
q8-12h q8-12h q24h q24h q24h q24h
*Oral and intravenous dosages are similar. Does not cross-react with the cortisol assay.
(also known as 21-hydroxlase deciency), which is discussed here. Several phenotypes of this disorder have been described. In 75% of cases, classic CYP21A2 deciency is manifested as a salt-wasting form, where impaired 21-hydroxylation of progesterone results in aldosterone deciency. In addition, impaired 21-hydroxylation of 17-hydroxyprogesterone results in cortisol deciency. The saltwasting form is manifested within the rst few months of life as hypovolemia, hyperkalemia, and hyperreninemia. Reduced negative feedback leads to an increase in ACTH secretion and accumulation of steroid hormone precursors upstream of CYP21A2. Shunting of precursors toward oxidation at carbon 17 generates an excess of adrenal androgens, which leads to ambiguous genitalia in newborn girls. In a minority of cases, CYP21A2 deciency is manifested as a simple virilizing form, in which aldosterone synthesis is intact. The diagnosis of CAH is made by biochemical screening for elevated plasma levels of 17-hydroxyprogesterone, followed by conrmatory genetic and provocative biochemical testing. Treatment centers around glucocorticoid and mineralocorticoid replacement, as well as surgical correction of genital anomalies in girls.9
result from ACTH-induced melanogenesis.10 Hormonal insufciency secondary to intrinsic adrenal disease arises from three general mechanisms: congenital adrenal dysgenesis/hypoplasia, defective steroidogenesis, and adrenal destruction. Of these, adrenal destruction from autoimmune causes is the most common, followed by infectious adrenalitis (tuberculous, fungal, or viral), adrenal replacement by metastatic tumor, and adrenal hemorrhage (Waterhouse-Friderichsen syndrome). The latter occurs in the setting of septicemia from meningococcus or other organisms and is more common in pediatric and asplenic patients.11
Secondary Adrenal Insufciency
Secondary adrenal insufciency is a relatively common disorder that results from ACTH deciency and often occurs in the setting of pharmacologic steroid withdrawal. Patients receiving high supraphysiologic doses of glucocorticoids (greater than the equivalent of 20 mg prednisone daily, Table 39-1) for more than 5 days and those receiving low supraphysiologic doses for more than 3 weeks are at risk for suppression of the HPA axis. Surgical cure of Cushings syndrome (see later) likewise results in glucocorticoid withdrawal. The rate of recovery from HPA axis suppression varies in accordance with the duration and severity of the previous glucocorticoid excess, and the need for glucocorticoid supplementation may last several years.12 Other less common causes of secondary adrenal insufciency include panhypopituitarism secondary to neoplastic or inltrative replacement, granulomatous disease, and pituitary hemorrhage/infarction. Pituitary infarction may occur in the setting of severe postpartum hemorrhage (Sheehans syndrome).
ADRENAL INSUFFICIENCY Primary Adrenal Insufciency (Addisons Disease)
Addison originally described 10 patients with anemia . . . feebleness of the heart action . . . [and] a peculiar change of color in the skin associated principally with tuberculous destruction of the adrenal glands. This rare disease is most commonly manifested as weakness and fatigue, anorexia, nausea or vomiting, weight loss, hyperpigmentation, hypotension, and electrolyte disturbances (hyponatremia and hyperkalemia). Hyperpigmentation, previously thought to be caused by elevated levels of POMC and its cleavage product -melanocytestimulating hormone, is now believed to
Adrenal Insufciency in the Critically Ill
A growing body of literature suggests that critically ill patients with sepsis or systemic inammatory response syndrome may be affected by acute reversible dysfunction of the HPA axis. The incidence of the disorder is
Chapter 39 The Adrenal Glands
1007
approximately 30% in critically ill patients, although this gure may be higher in those with septic shock. Whether these patients incur increased mortality because of adrenal insufciency remains to be dened. Proposed mechanisms of reversible HPA axis dysfunction include adrenal ACTH resistance and decreased responsiveness of target tissues to glucocorticoids. Glucocorticoid supplementation in septic patients has been the topic of at least 14 randomized, controlled trials. In these studies there appears to be an inverse relationship between survival benet and glucocorticoid dose, with physiologic (i.e., replacement) doses yielding a median relative survival benet of 1.33 and high supraphysiologic doses demonstrating signicant harm. Although the data remain controversial, the most recent evidence suggests that patients with vasopressordependent septic shock may benet from 5- to 7-day courses of glucocorticoids in the dose range of 400 mg/ day or less of hydrocortisone or equivalent.13
Suspected adrenal insufficiency
8 AM serum or salivary cortisol Serum cortisol 15 g/dL or salivary cortisol 5.8 ng/mL Serum cortisol >15 g/dL or salivary cortisol >5.8 ng/mL
Adrenal insufficiency possible
Adrenal insufficiency unlikely
250 g ACTH (cosyntropin) stimulation test Post cortisol <18 g/dL Post cortisol 18 g/dL
Adrenal Crisis
Acute adrenal insufciency, or adrenal crisis, is a lifethreatening condition that typically occurs in individuals with already marginal adrenocortical function who are subjected to a signicant acute physiologic stressor such as infection or trauma. Sudden complete loss of adrenal function, as occurs with Waterhouse-Friderichsen syndrome and certain hypercoagulable states, can also occur with adrenal crisis. Clinical ndings include shock, abdominal pain, fever, nausea and vomiting, electrolyte disturbances, and occasionally, hypoglycemia. Mineralocorticoid deciency resulting in an inability to maintain sodium and intravascular volume is the primary pathogenetic mechanism, although diminished cardiovascular responsiveness to catecholamines secondary to glucocorticoid deciency also plays a role. Treatment of adrenal crisis centers around large-volume (>2 L) intravenous (IV) resuscitation with isotonic saline and glucocorticoid administration in the form of either hydrocortisone (100 mg IV every 6-8 hours) or dexamethasone (4 mg IV every 24 hours). Dexamethasone is long acting and carries the advantage of not interfering with biochemical assays of endogenous glucocorticoid production. Ironically, mineralocorticoid replacement is not an early priority because the sodium- and uid-retentive effects of mineralocorticoids do not occur until several days after administration. Fluid and electrolyte balance can be rapidly achieved by saline infusion.14
Adrenal insufficiency possible Adrenal insufficiency unlikely
8 AM serum ACTH Increased ACTH Decreased or normal
Primary adrenal insufficiency
Secondary adrenal insufficiency
Figure 39-7 Algorithm for the diagnosis of adrenal insufciency. The adequacy of cortisol production is initially assessed with morning cortisol measurement. Patients with low or borderline values undergo provocative adrenocorticotropic hormone (ACTH) stimulation testing, with serum cortisol being measured before and 30 to 60 minutes after the administration of ACTH. Failure to mount a adequate response to ACTH establishes the diagnosis of adrenal insufciency in most cases. The cause of adrenal insufciency is then investigated with morning ACTH measurement.
Diagnosis and Treatment of Adrenal Insufciency
As is true for most endocrine disorders, diagnosis of adrenal insufciency depends on maintaining sufcient clinical suspicion for the disease. The clinical manifestations are discussed earlier. Surgeons are most likely to encounter patients with adrenal insufciency in the intensive care unit, the trauma suite, or the operating room when treating patients with steroid-dependent
chronic illnesses. Routine and provocative biochemical testing is necessary to conrm the diagnosis (Fig. 39-7). The rst step is to document inadequate cortisol production, which can be done by measuring morning levels of cortisol in serum or saliva. In most patients, a morning serum cortisol concentration greater than 15 g/dL or a morning salivary cortisol concentration greater than 5.8 ng/mL effectively excludes adrenal insufciency. These cutoff values are deliberately set high to maximize sensitivity at the expense of specicity for this screening test. Patients whose values fall below these thresholds undergo provocative testing with exogenous ACTH (cosyntropin). A high-dose cosyntropin stimulation test is performed by administering 250 g of cosyntropin and
1008
Section VIII Endocrine
Table 39-2
Appropriate Perioperative Glucocorticoid Regimens for Patients With Secondary Adrenal Insufciency Caused by Chronic Pharmacologic Steroid Use
DEGREE OF SURGICAL STRESS EXAMPLES DAILY GLUCOCORTICOID DOSE
Minor Moderate Major
Procedures under local anesthesia, most outpatient procedures, inguinal hernia repair Routine abdominal, peripheral, vascular, or orthopedic surgery Resection of gastrointestinal cancer, cardiopulmonary bypass
Hydrocortisone, 25 mg or equivalent Hydrocortisone, 50-75 mg or equivalent Hydrocortisone, 100-150 mg or equivalent
Adapted from Salem M, Tainsh RE Jr, Bromberg J, et al: Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg 219:416-425, 1994.
measuring serum cortisol levels 30 to 60 minutes later. A positive test (i.e., a stimulated cortisol level of less than 18 g/dL) is strongly suggestive of adrenal insufciency. Likewise, a normal test greatly reduces the likelihood of the diagnosis, although false-negative results may occur in patients with mild, new-onset, or secondary adrenal insufciency. Higher cutoff values (25 g/dL) have been recommended for evaluation of critically ill patients.15 After the diagnosis of adrenal insufciency has been made, a morning ACTH level is determined to differentiate between primary and secondary adrenal insufciency. Treatment of adrenal crisis is discussed earlier. The goal of maintenance therapy for chronic adrenal insufciency is to replace physiologic glucocorticoid and mineralocorticoid levels. Daily adult cortisol production is in the range of 10 to 20 mg, which can be replaced by the long-acting, orally bioavailable agent prednisone at a dose of 5 mg/day. Typical mineralocorticoid replacement consists of udrocortisone, 0.1 mg/day. Commensurate increased dosages of glucocorticoids are needed during periods of minor and major physiologic stress, such as mild infections (minor), as well as trauma, signicant infections, burns, or elective surgery (major).
DISEASES OF THE ADRENAL CORTEX Primary Hyperaldosteronism
Epidemiology and Clinical Features Primary hyperaldosteronism, or unregulated release of excess aldosterone from one or both adrenal glands, was rst described by Jerome Conn, an endocrinologist at the University of Michigan, in 1954. Primary hyperaldosteronism is classically manifested as resistant hypertension and hypokalemia, although recent reports have revealed that the majority of patients may be normokalemic, depending on the population screened. Hypokalemia is probably a manifestation of severe or late-stage disease. The prevalence of primary hyperaldosteronism has been the topic of considerable debate. Until recently it was generally believed to affect roughly 1% of patients with hypertension. Widespread application of the aldosterone-renin ratio, discussed later, as a screening test in certain centers led to reports of a 10% to 40% prevalence of primary hyperaldosteronism among hypertensives.17 There is some consensus that these higher gures reect strong referral bias and that the actual prevalence in unselected hypertensive patients is probably 7% or less. Nonselective use of the aldosterone-renin ratio to identify patients with primary hyperaldosteronism is known to signicantly decrease the fraction of patients with surgically correctable disease (unilateral aldosteronoma), although the absolute number of surgically treatable cases increases.18 The mean age at diagnosis of primary hyperaldosteronism is roughly 50, and the disease has a mild male predilection. Most patients are asymptomatic, although those with signicant hypokalemia may complain of muscle cramps, weakness, or paresthesias. Patients typically have moderate to severe hypertension that is refractory to medical therapy. It is common for them to require two to four antihypertensive medications. Responsiveness to spironolactone may be seen, a feature that is predictive of a good response to surgical treatment. Primary hyperaldosteronism is a potentially curable cause of signicant cardiovascular disease. A recent study comparing 124 subjects with biochemically conrmed primary hyperaldosteronism and hypertensive controls matched for age and systolic blood pressure revealed
Perioperative Steroid Administration
Recommendations concerning glucocorticoid administration during elective surgery have been based primarily on uncontrolled, retrospective studies. The need for supraphysiologic doses of glucocorticoids in this setting has generally been overstated. Patients with secondary adrenal insufciency as a result of chronic glucocorticoid treatment of autoimmune or inammatory conditions have a 1% to 2% risk for hypotensive crisis without perioperative glucocorticoid coverage. To prevent this rare but hazardous complication, chronic glucocorticoid users are, at the least, maintained on their usual glucocorticoid dosage throughout the perioperative period. Supplementation above this level is given in short courses according to the guidelines listed in Table 39-2.16 Patients undergoing unilateral adrenalectomy are given supplemental glucocorticoids only if the underlying diagnosis is Cushings syndrome.
Chapter 39 The Adrenal Glands
1009
Table 39-3
Causes of Primary Hyperaldosteronism*
SELECTIVE SCREENING NONSELECTIVE SCREENING
Aldosterone-producing adenoma Bilateral adrenal hyperplasia (idiopathic hyperaldosteronism) Aldosterone-producing adrenocortical carcinoma Familial hyperaldosteronism Type 1 (glucocorticoid-remediable aldosteronism) Type 2 (nonglucocorticoid-remediable aldosteronism)
60% 35% <1% <1% <1%
30% 65% <1% <1% <1%
*Rates of specic pathologies are highly dependent on the pattern of screening (selective versus nonselective).
that primary hyperaldosteronism is associated with a signicantly increased risk for stroke, myocardial infarction, atrial brillation, and left ventricular hypertrophy.19 These data add to existing evidence that the adverse cardiovascular sequelae of primary hyperaldosteronism are greater than those caused by blood pressure elevation alone. Successful removal of an aldosteronoma leads to regression of many of these adverse physiologic changes. The most common causes of primary hyperaldosteronism are unilateral aldosterone-producing adenoma (aldosteronoma) and bilateral adrenal hyperplasia (also termed idiopathic hyperaldosteronism, Table 39-3). In the past, aldosteronoma was present in more than 60% of cases, but that gure has decreased substantially as nonselective screening with the aldosterone-renin ratio has been applied. This phenomenon may reect increased detection of hyperplasia, which is characterized by milder biochemical abnormalities than occurs with aldosteronoma.
Biochemical Diagnosis and Localization The goal of diagnostic testing is to identify and lateralize aldosteronomas. There is some consensus that biochemical screening should be performed in all patients with hypertension and unexplained hypokalemia, as well as those with hypertension sufciently resistant to medical therapy to warrant investigation for secondary hypertension. Establishing the diagnosis of primary hyperaldosteronism begins with determining the ratio of plasma aldosterone to plasma renin activity (expressed here as ng/dL divided by ng/mLh, Fig. 39-8). This test is performed after discontinuation of interfering medications such as spironolactone, angiotensin-converting enzyme inhibitors, diuretics, and -adrenergic blockers. Variable cutoff values have been used in the literature, but a cutoff of 30 yields a sensitivity of approximately 90%.20 A subset of patients with essential hypertension will have suppressed renin levels, which may result in false elevations of the aldosterone-renin ratio. Thus, inclusion of an absolute aldosterone concentration of greater than 15 mg/dL increases the specicity of initial screening. Patients who test positive and are younger than 30 years are genetically screened for glucocorticoid-remediable aldosteronism (familial hyperaldosteronism type 1), especially if they have a family history of early-onset hypertension. This
rare autosomal dominant condition results in abnormal regulation of aldosterone synthesis by ACTH and can be treated medically. Conrmatory biochemical testing is aimed at demonstrating inappropriately high (nonsuppressible) aldosterone levels by creating a state of hypervolemia/sodium excess. This is done with IV saline loading (2-3 L of isotonic saline given over a 4- to 6-hour period, followed by measurement of plasma aldosterone) or oral salt loading (200 mEq = 5000 mg sodium daily over a 3-day period, followed by measurement of 24-hour urine aldosterone excretion). Some centers administer high-dose udrocortisone (0.1 mg every 6 hours) during oral salt loading to increase the specicity of suppression testing, but this method has not been widely adopted. After the diagnosis has been conrmed, localization is performed with anatomic imaging, selective venous sampling, and sometimes functional scanning. The fact that most aldosteronomas are smaller than 15 mm in maximum dimension poses some challenge to localization. Thin-cut (3 mm) adrenal computed tomography (CT) is the preferred initial localization test (Fig. 39-9). In patients younger than 40 years, the nding of a solitary adrenal mass 1 cm or greater in size and a normal contralateral adrenal gland is sufcient to proceed to surgery. Unilateral adrenalectomy yields successful clinical outcomes in approximately 95% of such cases. The next step in the localization algorithm is selective adrenal venous sampling. This test relies on simultaneous measurement of cortisol and aldosterone levels in the peripheral circulation, as well as the left and right adrenal veins (Fig. 39-10). Greater than a vefold elevation in cortisol concentration in a sample relative to peripheral blood indicates successful cannulation of an adrenal vein (positive control). Lateralization is indicated by an unbalanced ratio of aldosterone to cortisol in the left and right adrenal veins, with a fourfold greater ratio on one side than on the other identifying the culprit gland. Considerable controversy exists over which patients undergo this study, an invasive procedure with a 90% technical success rate in experienced hands.21 There is consensus that adrenal venous sampling should be applied in all cases in which the biochemical diagnosis of primary hyperaldosteronism has been conrmed and thin-cut adrenal CT reveals either no abnormalities or bilateral abnormalities. Of the remaining patients who have a unilateral mass on CT, a small but not insignicant fraction (2%-10%) will
1010
Section VIII Endocrine
Ratio <30 Biochemical diagnosis
PAC/PRA Ratio >30
Discontinue interfering medications*
Suppression of urine aldosterone
24-hr urine free aldosterone Urinary potassium excretion Oral or IV sodium loading No suppression Probable primary hyperaldosteronism Unilateral adrenal mass >1 cm, normal contralateral adrenal, age 40 years
Thin cut adrenal CT Nonlateralizing CT Consider for age >40 years Consider repeat testing Failed study Adrenal vein sampling
Localization
Nonlateralizing (bilateral adrenal hyperplasia)
Lateralizing (aldosteronoma)
Management
Observation, consider repeat testing if suspicion remains
Medical management
Adrenalectomy
*Including spironolactone, ACE inhibitors, diuretics, -blockers.
Figure 39-8 Algorithm for diagnosis, localization, and management of primary hyperaldosteronism. PAC, plasma
aldosterone concentration in ng/dL; PRA, plasma renin activity in ng/mLh. Initial screening with the PRA/PAC ratio is performed, followed by conrmatory testing with sodium loading. After the biochemical diagnosis has been established, noninvasive localization is attempted with CT. Patients with clear CT evidence of a unilateral abnormality can proceed to adrenalectomy with a greater than 90% cure rate. Adrenal vein sampling is performed in patients with equivocal CT ndings and older patients, especially those older than 60, because nonfunctional cortical adenomas are found in 4% or more of this population and can cause false-positive CT localization.
represent false-positive localization and have persistent hyperaldosteronism after unilateral adrenalectomy. In these patients the adrenal mass represents a nonfunctioning cortical adenoma and the true underlying diagnosis is either a contralateral microaldosteronoma or bilateral adrenal hyperplasia, the latter of which is not surgically remediable. Because patients 40 years or older are more likely to have nonfunctioning adrenal cortical adenomas, some authors have advocated adrenal venous sampling in all older patients. Yet others have recommended universal application of this test in the workup of primary hyperaldosteronism. Practically speaking, approximately 20% to 30% of patients being evaluated for primary hyperaldosteronism undergo adrenal venous sampling when it is applied to select patients. The utility of the test is
limited by its low success rate in most reports (40%-80%), with the most common reason for incomplete adrenal venous sampling being failure to cannulate the right adrenal vein. Frequently, however, sufcient lateralizing information is provided during adrenal venous sampling to guide surgical treatment, even when the study is not bilaterally selective.22 Functional scanning with radiolabeled 131I-6--iodomethylnorcholesterol (NP-59) may be considered as a third-line localization test for patients without conclusive lateralizing information on either CT or adrenal venous sampling. The sensitivity of NP-59 scanning is low in small tumors. Most aldosteronomas are small to begin with, and those with negative CT scans represent the smallest subset of tumors. For these reasons, NP-59 scanning does not generally aid management in primary hyperaldosteronism.
Chapter 39 The Adrenal Glands
1011
Surgical Management and Outcomes Laparoscopic adrenalectomy is the preferred procedure for the management of aldosteronoma and most other adrenal tumors.23 Cure of primary hyperaldosteronism is dened by clinical and biochemical end points. Reductions in blood pressure, antihypertensive medication requirements, and plasma/urine aldosterone levels and resolution of hypokalemia (if previously present) are observed as soon as 24 hours after successful surgery. Overall cure rates range from 75% to 95% at subspecialty centers, depending on the specic criteria for cure that are used. In general, more than 80% of patients can expect either normalization of blood pressure or a signicant reduction in antihypertensive medication requirements (typically from three to four medications down to one). In some patients, depending on the degree of preoperative sodium overload, blood pressure may take several weeks to improve. Our practice is to stop all antihypertensive medications immediately after surgery, with the exception of -blockers, which must be tapered to avoid a rebound phenomenon. In patients who continue to be hypertensive in the short term, medications may be added back temporarily as needed until blood pressure gradually reaches a new equilibrium over time. A subset of patients with the following preoperative features demonstrates reduced benet from surgical treatment: male, age older than 45 years, family history of hypertension, long-standing hypertension, and no response to spironolactone.24 These characteristics indicate a component of essential hypertension and, in some cases, irreversible cardiovascular alterations resulting from chronic disease. Based on these features, patients need to be counseled about what they can expect to gain from surgery.
B
Figure 39-9 Appearance of aldosteronoma on anatomic imaging. A, Venous phase, contrast-enhanced CT demonstrating a 2-cm left aldosteronoma. B, Late arterial phase, coronal CT demonstrating a 1.7-cm left aldosteronoma (arrow) and a normal right adrenal gland (arrowhead).
Cushings Syndrome
Epidemiology and Clinical Features The clinical features of glucocorticoid excess were rst documented by Harvey Cushing in 1912. He described a young woman of extraordinary appearance in whom obesity, hirsutism, amenorrhea, easy bruising, and extreme muscle weakness developed. The principal differential diagnosis to be considered when evaluating patients for Cushings syndrome is obesity, an increasingly common condition. A subset of signs and symptoms, including easy bruising, muscle weakness, hypertension, plethora (a red facial appearance caused by thinning of the skin), and hirsutism, may allow discrimination between Cushings syndrome and obesity based on clinical features (Fig. 39-11). The most common cause of Cushings syndrome is pharmacologic glucocorticoid use for the treatment of inammatory disorders. Endogenous Cushings syndrome is rare, with 5 to 10 individuals affected per million. Of these, the majority of affected individuals (75%) will have Cushings disease, that is, glucocorticoid excess caused by an ACTH-hypersecreting pituitary adenoma. The remainder will be split between primary adrenal Cushings syndrome (15%) and ectopic ACTH syndrome (<10%), the latter of which most
commonly arises from either neuroendocrine tumors or bronchogenic malignancies in the thorax. Cushings syndrome is a lethal disease. The physiologic derangements resulting from glucocorticoid excess, including hypertension (present in >70% of cases), hyperglycemia, and truncal obesity, ultimately yield a vefold excess in mortality, primarily secondary to cardiovascular complications.25 Thus, all efforts need to be made to identify and appropriately treat patients with Cushings syndrome.
Biochemical Diagnosis and Localization Diagnosis of Cushings syndrome relies on demonstration of inappropriate cortisol secretion or loss of physiologic negative feedback. Normally, cortisol release follows a predictable circadian rhythm, with the peak reached approximately 1 hour after waking and the nadir around midnight. Thus, inappropriate cortisol secretion can be detected as either elevated cortisol release over a 24-hour period or a higher than expected level in the late evening.
1012
Section VIII Endocrine
Right: Cortisol 328 Aldosterone 13 A/C ratio = 0.04
Left: Cortisol 275 Aldosterone 4414 A/C ratio = 16
Right: Cortisol 1201 Aldosterone 2646 A/C ratio = 2.2
Left: Cortisol 1996 Aldosterone 3897 A/C ratio = 2.0
Peripheral: Cortisol 44 Aldosterone 72
Peripheral: Cortisol 64 Aldosterone 57
Right: Cortisol 33 Aldosterone 29 A/C ratio = ?
Left: Cortisol 204 Aldosterone 452 A/C ratio = 2.2
Peripheral: Cortisol 43 Aldosterone 27
C
Figure 39-10 Possible outcomes of adrenal vein sampling for primary hyperaldosteronism. Aldosterone is expressed in ng/dL, cortisol in g/dL. A, Successful study lateralizing strongly to the left adrenal. B, Successful study, nonlateralizing. Stimulation with adrenocorticotropic hormone yielded high adrenal vein cortisol levels. C, Failed study. The right adrenal vein was not cannulated.
Traditionally, lack of negative feedback has been assessed by dexamethasone suppression testing and other types of provocative tests, many of which are cumbersome and require inpatient hospitalization. The recent advent of late evening salivary cortisol testing has provided an attractive and feasible alternative to suppression testing. More than 90% of circulating cortisol is bound to plasma proteins. Unbound cortisol can be detected in urine and saliva, and assessment of these body uids forms the basis of biochemical screening for Cushings syndrome (Fig. 39-12). Twenty-four-hour urine collection for determination of urinary free cortisol is performed at least twice for initial screening. Unequivocally elevated levels prompt immediate further testing to determine the cause/subtype of Cushings syndrome (i.e., primary
adrenal cause versus a pituitary cause versus ectopic ACTH syndrome). Patients with moderately elevated 24hour urinary cortisol levels undergo conrmatory testing with two late evening (bedtime) cortisol measurements. A high cutoff value of 550 ng/mL carries a sensitivity of 93% and a specicity of 100%.26 Primary adrenal Cushings syndrome, also termed ACTH-independent Cushings syndrome, is caused by autonomous adrenal cortisol production and is therefore generally associated with an undetectable ACTH level (<5 pg/mL) because of feedback inhibition. The underlying pathology is variable, with a solitary adrenal adenoma found in approximately 90% of cases, adrenocortical carcinoma in less than 10%, and bilateral micronodular or macronodular hyperplasia in less than 1%. Nearly all
Chapter 39 The Adrenal Glands
1013
A B
C
Figure 39-11 Clinical manifestations of Cushings syndrome. A, Moon facies, plethora, and excess supraclavicular
fat in a woman with Cushings syndrome. B, Buffalo hump in a woman with Cushings syndrome. C, Purple abdominal striae in a man with Cushings syndrome.
these lesions, except micronodular hyperplasia, are readily apparent on CT.27 Hypercortisolemia associated with normal or elevated ACTH levels is indicative of ACTH-dependent Cushings syndrome, which is most commonly caused by a pituitary corticotroph microadenoma (Cushings disease). Suspicion of ACTH-dependent Cushings syndrome prompts pituitary imaging and high-dose dexamethasone suppression testing (i.e., serum or urine cortisol measurement after the administration of 2 mg of dexamethasone every 6 hours over a 48-hour period). Dexamethasone is chosen because it does not cross-react with present biochemical assays for cortisol. Corticotroph adenomas are commonly suppressed in response to high-dose dexamethasone administration, whereas ectopic ACTH sources are completely lacking in feedback inhibition. Slightly more than half of corticotroph microadenomas are visible on pituitary magnetic resonance imaging (MRI). Detection of a
pituitary mass larger than 6 mm in diameter in a patient with ACTH-dependent Cushings syndrome that is suppressed with high-dose dexamethasone justies proceeding to pituitary surgery.28 In the absence of a demonstrable mass, bilateral inferior petrosal sinus ACTH sampling with CRF stimulation is pursued. Demonstration of a central-to-peripheral ACTH gradient in a study performed by a skilled practitioner is sufcient to diagnose Cushings disease. Absence of a clear gradient prompts CT imaging of the chest/abdomen and occasionally somatostatin receptor scintigraphy to identify an ectopic ACTH source.
Surgical Management and Outcomes Perioperative and postoperative glucocorticoid administration is obviously essential in the care of patients with Cushings syndrome. For patients undergoing adrenalectomy for Cushings syndrome, perioperative stress dose
1014
Section VIII Endocrine
Normal Biochemical diagnosis
24-hr urine free cortisol 1-3 normal Late evening salivary cortisol 2 <550 ng/dL
>3-fold elevation
>550 ng/dL
Cushings syndrome unlikely
Probable Cushings syndrome
Undetectable ACTH-independent disease
ACTH
Detectable ACTH-dependent disease
CT of adrenals Localization
Mass >6 mm Suppression
Pituitary MRI, high-dose dexamethasone suppression test No mass Bilateral inferior petrosal sinus sampling
Gradient detected
No gradient
Chest/Abd CT, somatostatin receptor scintigraphy
Management
Adrenalectomy 90% effective
Transsphenoidal pituitary microsurgery 75% effective Failure Bilateral adrenalectomy
Resection
Ectopic ACTH (<10%)
Primary adrenal Cushings syndrome (15%)
Cushings disease (75%)
Figure 39-12 Algorithm for the diagnosis, localization, and management of endogenous Cushings syndrome. A biochemical diagnosis can be established with either an unequivocally elevated 24-hour urine free cortisol level (greater than a threefold elevation) or an elevated late evening salivary cortisol. Most cases of Cushings syndrome are caused by Cushings disease (pituitary corticotroph microadenoma), in which plasma adrenocorticotropic hormone (ACTH) is elevated. An undetectable ACTH level establishes the diagnosis of ACTH-independent Cushings syndrome and prompts adrenal imaging. Bilateral adrenalectomy is considered for patients with Cushings disease not cured by transsphenoidal surgery.
steroids (hydrocortisone, 100 mg IV every 8 hours for 24 hours) are recommended. In the most common scenario of resection of a solitary adrenal Cushings adenoma, steroids can usually be tapered to physiologic replacement levels over the course of several weeks. However, a subset of patients with Cushings syndrome of greater duration and severity will have lasting HPA axis suppression that requires glucocorticoid supplementation for longer periods, sometimes for more than 1 year. It is our
practice to routinely administer perioperative antibiotics for 24 hours to patients undergoing adrenalectomy for Cushings syndrome because of their elevated risk for surgical site infection. Management of patients who undergo pituitary surgery for Cushings disease is variable. In some centers, glucocorticoids are withheld during the immediate postoperative period to provide a window during which early remission may be assessed.29 A subnormal morning
Chapter 39 The Adrenal Glands
1015
cortisol level on postoperative day 1 or 2 is indicative of cure. Glucocorticoid supplementation is then resumed, usually for at least 6 months, until the HPA axis recovers. Because of the signicant risk for postoperative adrenal crisis in patients with Cushings syndrome of all subtypes, glucocorticoid supplementation is ideally managed in conjunction with an experienced endocrinologist. Adrenalectomy is more than 90% effective in the treatment of primary adrenal Cushings syndrome. Failures may result from local and occasionally distant tumor recurrence in the case of malignant disease. Pituitary microsurgery for Cushings disease, typically performed via a transnasal transsphenoidal approach, is approximately 75% successful in experienced hands. Remission rates may be improved by reoperation or pituitary irradiation in patients whose basal cortisol levels do not fall appropriately after initial surgery. Laparoscopic bilateral adrenalectomy is considered for patients in whom pituitary surgery has failed.30
Special Case: Subclinical Cushings Syndrome The term subclinical Cushings syndrome has been used to describe patients with incidentally discovered adrenal masses (see The Incidentally Discovered Adrenal Mass, later) who display biochemical evidence of cortisol hypersecretion without overt signs or symptoms of Cushings syndrome. This disease entity has been incompletely characterized with respect to its physiologic consequences and natural history. Clear-cut denitions for the diagnosis of subclinical Cushings syndrome, such as cutoff values for biochemical tests and objective assessment guidelines for the presence or absence of clinical features, are lacking. Some reports indicate that hypertension, dyslipidemia, and impaired glucose tolerance are highly prevalent in individuals with subclinical Cushings syndrome.31 However, adrenalectomy for this entity has not been consistently demonstrated to yield health benets. Progression to overt Cushings syndrome occurs in less than 10% of cases. Thus, at present, patients found to have subclinical hypercortisolism are monitored for the development of adverse cardiovascular and metabolic features. Therapy is primarily medical and directed at the subset of patients with progressive disease. However, lower biochemical thresholds for surgical treatment need to be considered for larger tumors (i.e., those 3-4 cm in diameter).
Figure 39-13 CT demonstrating a 10-cm left adrenocortical carcinoma. Note the areas of central necrosis.
biochemically detected by measurement of 24-hour urine testosterone, DHEA, and DHEA-S. Although laparoscopic adrenalectomy remains the preferred procedure for the majority of sex steroidsecreting tumors, the high probability of malignancy merits close radiographic and intraoperative inspection for evidence of invasion or metastasis, or both. Open adrenalectomy is performed for malignant tumors.
Adrenocortical Carcinoma
Adrenocortical carcinoma is a rare tumor with an annual incidence of approximately one per million. Almost all cases occur in patients 40 to 50 years of age, although there is a minor peak in children younger than 5. Adrenocortical carcinomas demonstrate no signicant gender predilection. They are frequently very large at initial evaluation (mean tumor size, 9-12 cm), and they generally carry a poor prognosis. Historically, 5-year survival rates have been in the 15% to 20% range, although improved survival rates of 25% to 60% have been reported during the past several years.33 More than half of adrenocortical carcinomas are functional. Cushings syndrome is most commonly seen, followed by virilization. Radiographic evaluation is primarily performed with CT, which typically reveals a heterogeneous mass with irregular/ indistinct borders, central necrosis, and invasion of adjacent structures (Fig. 39-13). Metastases to the lymph nodes, liver, and lungs may be found. Treatment of adrenocortical carcinoma centers around radical open surgery. Complete resection can be achieved in up to 70% of patients in experienced hands. Management frequently involves en bloc resection of adjacent organs or regional lymphadenectomy (or both). Particular care must be taken when dealing with right-sided adrenocortical carcinomas larger than 9 cm because direct tumor extension into the inferior vena cava and some-
Sex Steroid Excess
Adrenal tumors causing clinical features of sex steroid excess are rare. The majority of such tumors are virilizing (as opposed to feminizing), and they may initially be seen at a late stage in association with an advanced adrenal malignancy. Virtually all feminizing tumors are malignant, whereas approximately a third of virilizing tumors are malignant. Of adrenocortical carcinomas, 20% cause virilization, with the majority of these cases occurring in children. An additional 24% of adrenocortical carcinomas will display mixed features of Cushings syndrome and virilization.32 Virilizing tumors may be
1016
Section VIII Endocrine
times the right heart may be observed. Tumors demonstrating intravascular extension may need to be resected while the patient is on cardiopulmonary bypass to reduce the likelihood of lethal intraoperative tumor embolization. Patients who undergo incomplete resection of adrenocortical carcinomas have extremely limited life expectancy (median survival, <1 year). Even those who undergo successful surgery are prone to the development of local recurrence and metastases, which typically occur within 2 years. The principal chemotherapeutic agent for the treatment of adrenocortical carcinoma is mitotane (o,p-DDD, or 1,1-dichloro-2-[o-chlorophenyl]-2-[p-chlorophenyl] ethane), a derivative of the insecticide DDT that is a direct adrenocortical toxin. Mitotane has been used clinically both as an adjuvant to surgery and as primary therapy in individuals with unresectable or metastatic disease. The rarity of adrenocortical carcinomas has made systematic assessment of mitotanes efcacy difcult. On balance, mitotane treatment does not appear to improve survival in patients who have undergone complete resection, but it may offer a moderate survival advantage in patients with unresectable or metastatic disease.34 Its use is limited by signicant gastrointestinal and neurologic toxicity. Conventional cytotoxic chemotherapy and external beam radiation therapy currently play little role in the management of adrenocortical carcinoma, although some efcacy has been demonstrated with cisplatinbased regimens.
to have the disease.36 The differential diagnosis of pheochromocytoma is extensive and encompasses such diverse processes as hyperthyroidism, hypoglycemia, coronary artery disease, heart failure, stroke, drug-related effects, and panic disorder. Pheochromocytoma has been described as a biologic time bomb because of the potentially lethal cardiovascular effects of the bioactive compounds secreted by these tumors. Thus, despite the challenges in diagnosis, clinicians need to aggressively screen for this disease and seek appropriate treatment for affected patients. Previously, pheochromocytoma was dubbed the 10% tumor for the reason that 10% are bilateral, 10% malignant, 10% extra-adrenal, and 10% familial. Recent discoveries regarding the genetic underpinnings of pheochromocytoma have challenged these old axioms.37
DISEASES OF THE ADRENAL MEDULLA Pheochromocytoma
Epidemiology and Clinical Features The rst account of pheochromocytoma was published in 1886 by Felix Frankel, who described a young woman suffering from intermittent attacks of palpitations, anxiety, vertigo, and headache. Autopsy revealed bilateral adrenal tumors that stained brown when treated with chromium salts. The characteristic positive chromafn reaction lends these adrenomedullary tumors the name pheochromocytoma (dusky-colored tumor from the Greek phaios, or dusky). Successful surgical management of pheochromocytoma was initially described in 1926 by both Csar Roux and Charles Mayo.35 Pheochromocytoma affects approximately 0.2% of hypertensive individuals. Males and females are affected equally. The peak incidence in sporadic cases lies between the ages of 40 and 50, whereas familial cases tend to be manifested earlier. A subset of patients have the classic triad of headache, diaphoresis, and palpitations, although almost all patients will display at least one of these symptoms. Hypertension is present in 90% of cases and may be episodic or sustained. The principal challenge in making the diagnosis of pheochromocytoma arises from the fact that essential hypertension is common and the clinical features suggestive of pheochromocytoma are nonspecic. In fact, only 0.5% of patients with hypertension and suggestive features will ultimately prove
Biochemical Diagnosis and Localization Establishment of a biochemical diagnosis of pheochromocytoma rests on detection of elevated levels of catecholamines and their metabolites in body uids. Measurement of 24-hour urine levels of these compounds has long been the cornerstone of biochemical testing, and they remain the most reliable tests available today. In 2002, measurement of free (unconjugated) metanephrines in plasma was introduced as an alternative screening tool for pheochromocytoma.8 Plasma free metanephrine testing carries an extremely high sensitivity that approaches 99% and, being a one-time blood test, is more convenient than 24-hour urine testing. However, the specicity of plasma free metanephrine testing is 89% at best, with specicities at most laboratories likely to lie in the 85% range or below. Given that pheochromocytoma is a rare diagnosis that is sought within a large pool of hypertensive individuals, false-positive test results are a major problem. In fact, it is estimated that false-positive tests outnumber true-positive tests as much as 30 to 1 when plasma free metanephrine testing is used as a principal screening tool.38 Therefore, the primary utility of plasma free metanephrine testing is to exclude pheochromocytoma when the test is negative (Fig. 39-14). When positive, conrmatory testing with 24-hour urinary levels of catecholamines and their metabolites is required. Many drugs and conditions, including sympathomimetics (present in many cold remedies), phenoxybenzamine (frequently initiated when suspicion of pheochromocytoma is raised), acetaminophen (which interferes with the plasma free metanephrine assay), many psychotropic drugs (notably tricyclic antidepressants), and major physical or psychological stressors, are capable of confounding catecholaminebased testing, thus further contributing to the problem of false-positive results. Tests performed during episodes of acute pain, critical illness, or urgent hospitalization may be misleading. The presence of confounding factors, including manifestations or treatment of competing diagnoses, is extremely common in the population being screened. Clearly, biochemical testing is ideally performed when the patient is as free as practically possible of all confounding factors.
Chapter 39 The Adrenal Glands
1017
Negative
Plasma free metanephrines Positive
Discontinue interfering medications*
Biochemical diagnosis
24-hr urine metanephrines and catecholamines Negative/equivocal Negative Repeat 24-hr urine metanephrines and catecholamines
Positive
Positive
Normetanephrine suppressed
Mild to moderate elevation Normetanephrine NOT suppressed Clonidine suppression test
Age <50 years or suggestion of multifocality CT/MRI Localization
MIBG
Age >50 years, single site
Management
Observation, consider repeat testing if suspicion remains
Preoperative conditioning
Surgery *Including sympathomimetics, phenoxybenzamine, acetaminophen, many psychotropic drugs.
Figure 39-14 Algorithm for the diagnosis, localization, and management of pheochromocytoma. Initial plasma free
metanephrine testing can effectively exclude the diagnosis if negative. Twenty-four-hour urine collection for catecholamines and their metabolites is generally performed twice, with cutoffs approximately twice the upper limit of normal being criteria for positivity (see Table 39-4). Clonidine suppression testing can be used for the small fraction of patients in whom the diagnosis remains uncertain after urine testing. Localization with CT or MRI follows biochemical conrmation of the diagnosis, with MIBG performed for younger patients and those otherwise at risk for multifocal disease. Phenoxybenzamine is given in escalating doses for at least 2 weeks before surgery.
The operating characteristics of catecholamine-based plasma and urine tests are listed along with corresponding cutoff values in Table 39-4. Cutoff values for 24-hour urine tests are deliberately set high to maximize specicity; in fact, these values are approximately double the upper 95% reference range in most laboratories. A urine collection may be considered positive if total metanephrines or any single catecholamine fraction (epinephrine, norepinephrine, or dopamine) is elevated above its cutoff value. This approach maintains high specicity and yields an acceptable sensitivity of 88%.38 Importantly, it takes into account the fact that pheochromocytomas both synthesize and metabolize catecholamines and that tumors may possess heterogeneous secretory proles, depending on their relative expression of synthetic and degradative enzymes (see Fig. 39-6).
Two 24-hour urine collections for catecholamines and their metabolites are sufcient to make (or exclude) the diagnosis of pheochromocytoma in almost all cases. Clonidine suppression testing, or measurement of plasma free normetanephrine levels after the oral administration of 0.3 mg of clonidine, may help clarify equivocal test results. Anatomic localization may be performed with MRI or CT. MRI is slightly more sensitive, but CT often yields better anatomic denition for operative planning (Fig. 39-15). Scintigraphy with 131I- or 123 I-labeled metaiodobenzylguanidine (MIBG, Fig. 39-16) is performed in select patients in whom multifocal disease is suspected. MIBG scanning is highly specic for pheochromocytoma but carries a sensitivity of only 77% to 90%. Positron-emission tomography (PET) with the use of novel 18F-labeled catecholamine
1018
Section VIII Endocrine
Table 39-4
TEST*
Cutoff Values for the Biochemical Diagnosis of Pheochromocytoma
CUTOFF VALUE (mol) CUTOFF VALUE (g) DEFINITIONS SENSITIVITY (%) SPECIFICITY (%)
Plasma free metanephrine Plasma free normetanephrine Urinary total metanephrines Urinary epinephrine Urinary norepinephrine Urinary dopamine Urinary total metanephrines and catecholamines
0.3 nmol/L 0.6 nmol/L 6.6 mol/day 191 nmol/day 1005 nmol/day 4571 nmol/day
59 g/L 110 g/L 1.3 mg/day 35 g/day 170 g/day 700 g/day
Paired test, positive if either or both values are elevated
99 71 29 50 8
85-89 99.6 99.6 99.6 100 99
Grouped test, positive if any one of following three urinary values are elevated: total metanephrines, epinephrine, norepinephrine, dopamine 7.9 mg/day 112 g/L Positive result = elevated level after clonidine and fall of less than 40
88
Urinary vanillylmandelic acid Clonidine suppression test Plasma free normetanephrine
40 mol/day 0.61 nmol/L
64 96
95 100
*When performed twice, 24-hour urine testing of urinary total metanephrines and catecholamines (grouped test) is both highly sensitive and highly specic.
analogues is probably more sensitive but remains largely investigational.39
Perioperative Care Throughout the rst half of the 20th century, perioperative mortality rates in the treatment of pheochromocytoma ranged from 26% to 50%. At the new millennium, the mortality rate in most specialty centers is approximately 2%. This dramatic improvement can largely be ascribed to advances in pharmacology, physiology, anesthesia, and perioperative medical care. The adverse perioperative hemodynamic changes that are most commonly observed with pheochromocytoma are intraoperative hypertension and postoperative hypotension. Intraoperative hypertension may be caused by stimulation of catecholamine release by anesthetic induction agents, as well as direct manipulation of the tumor. Postoperative hypotension may be profound. It results from a state of hypovolemia created by the presence of excess circulating catecholamines. Sudden withdrawal of this stimulus after tumor removal leads to peripheral arteriolar vasodilation, in addition to a dramatic increase in venous capacitance, which together may precipitate cardiovascular collapse. In their early report of a large, successful case series, investigators at the Mayo Clinic described the use of intraoperative -adrenergic blockade, followed by aggressive volume repletion and administration of -adrenergic agonists in the immediate postoperative period.40 The principles of contemporary perioperative care remain much the same. As soon as the biochemical diagnosis of pheochromocytoma has been conrmed, -adrenergic blockade is initiated to protect against hemodynamic lability. Our practice is to start with phenoxybenzamine, 10 mg twice daily. The dosage can
be titrated upward every 2 to 3 days to a maximum of 40 mg three times daily to achieve normalization of heart rate and blood pressure. The period of preoperative conditioning lasts at least 2 weeks to allow adequate reversal of -adrenergic receptor down-regulation. This restores sensitivity to vasopressor agents, which can then be used to treat the patient postoperatively. Phenoxybenzamine is a nonspecic, noncompetitive (irreversible), long-acting (half-life of 24 hours) -adrenergic antagonist. Although its use is associated with the side effects of postural hypotension and signicant nasal congestion, it is generally favored over 1-selective agents such as prazosin and doxazosin. Nasal congestion can actually serve as a useful indicator of adequate blockade. Furthermore, phenoxybenzamine provides the most complete -blockade among available agents, and its pharmacokinetics permits serum drug levels to decay in parallel with catecholamine levels postoperatively. -Blockers may be administered after adequate blockade has been achieved in the subset of patients with persistent tachycardia. -Blockers are never the rst agent administered because a decrease in peripheral vasodilatory -receptor stimulation results in unopposed adrenergic tone, which may exacerbate hypertension. Preoperative volume expansion with isotonic uids has been advocated in the past. However, in our experience the need for volume expansion is signicantly reduced when aggressive preoperative -blockade has been achieved because the resultant increase in venous capacitance restores euvolemia. Clinical suspicion of hypovolemia needs to remain high in the postoperative period, and patients need to be aggressively resuscitated if they become hypotensive or oliguric. Some patients may require vasopressors after tumor removal, especially if preoperative -blockade is incomplete.
Chapter 39 The Adrenal Glands
1019
C
Figure 39-15 Appearance of pheochromocytoma on anatomic imaging. A, Venous phase, contrast-enhanced CT scan demonstrating a right adrenal pheochromocytoma. The heterogeneity in the inferior vena cava represents swirling of contrast, not tumor thrombus or invasion. B, Coronal T2-weighted MRI demonstrating a left adrenal pheochromocytoma with central cystic change. C, Left anterior oblique magnetic resonance angiographic reconstruction demonstrating a right adrenal pheochromocytoma.
Surgical Management and Outcomes Successful operative treatment of pheochromocytoma is dependent on close communication between the surgeon and anesthesiologist. Needless to say, invasive hemodynamic monitoring is required, and uid management must be meticulous. Manipulation of the tumor needs to
be minimized, and the anesthetic team must be prepared to administer supplemental IV - and -blockers, as well as vasopressors, when necessary. Surgery is curative in greater than 90% of pheochromocytoma cases. Although these tumors are highly vascular and tend to adhere to adjacent structures (Fig.
1020
Section VIII Endocrine
B
123 I-MIBG scan of the abdomen demonstrating an isolated left adrenal pheochromocytoma. Physiologic radiotracer uptake is noted in the liver, right colon, and transverse colon. B, Whole-body 131I-MIBG scan demonstrating a large left para-aortic extra-adrenal pheochromocytoma. Physiologic radiotracer uptake is noted in the liver, salivary glands, and bladder. C, 131I-MIBG scan of the abdomen demonstrating malignant pheochromocytoma with local recurrence in the left adrenal bed and liver metastases.
Figure 39-16 Appearance of pheochromocytoma on functional imaging (MIBG scanning). A,
39-17), the great majority of them can be removed successfully via a laparoscopic approach. Laparoscopic resection is contraindicated when preoperative imaging demonstrates local invasion. Advances in surgical technique have resulted in reduced operative complication rates. Specically, functional image-guided focused exploration has replaced bilateral adrenal and retroperitoneal exploration and has led to diminished rates of solid organ injury.41
Molecular Genetics of Pheochromocytoma A number of recent reports describing novel germline mutations have demonstrated that familial pheochromocytoma is much more common than previously thought. Before the year 2000, pheochromocytoma was known to be associated with multiple endocrine neoplasia type 2
syndromes (40%-50% penetrant), von Hippel-Lindau syndrome (10%-20% penetrant), and neurobromatosis type 1 (1%-5% penetrant). The discovery that neuroendocrine cells of the carotid body proliferate in response to hypoxic stimuli led to the identication of mutations in the succinate dehydrogenase gene family in kindreds affected with pheochromocytoma/paraganglioma. Succinate dehydrogenase, which is made up of four subunits, is localized to the mitochondria and catalyzes essential steps in oxidative phosphorylation. Germline mutations in the B and D subunits, which are inherited in an autosomal dominant fashion, have been identied in approximately 10% of apparently sporadic pheochromocytoma cases.42 Thus, there is new consensus that 21% to 30% of pheochromocytomas are familial. Familial cases occur at an earlier age and are
Chapter 39 The Adrenal Glands
1021
vival rates at 5 years range from 20% to 45%. No histopathologic criteria for determining malignancy have demonstrated the ability to accurately predict the clinical course. Thus, malignancy is dened by the development of metastases (i.e., tumor implants distant from the primary mass in locations where neuroectodermal tissue is not normally found). The latter criterion distinguishes metastatic disease from possible multifocal primary disease. The most common sites of metastasis are the axial skeleton, lymph nodes, liver, lung, and kidney. Treatment of both primary and recurrent disease centers on surgical resection, which even in the absence of cure has signicant palliative benets.44 Malignant pheochromocytomas are minimally responsive to radiotherapy and chemotherapy. High-dose 131I-MIBG radionuclide therapy has been used to treat malignant and metastatic pheochromocytoma. Moderate initial response has been observed, but long-term benet is uncommon. Chronic medical management of catecholamine excess is performed with 1-selective blockers because of their favorable side effect prole.
THE INCIDENTALLY DISCOVERED ADRENAL MASS (INCIDENTALOMA) Epidemiology and Differential Diagnosis
Incidentally discovered adrenal masses, also termed clinically inapparent adrenal masses or incidentalomas, are discovered through imaging performed for unrelated/ nonadrenal disease. Their existence as a clinical entity is a by-product of advanced medical imaging. Incidentalomas were rst described in the early 1980s, when CT scanners became more prevalent in developed nations, and they have become a common clinical problem as the use of CT and MRI has become widespread. Incidentalomas are found in 2.1% of autopsies and 1% to 4% of abdominal imaging studies.45 The prevalence rises to greater than 4% in patients older than 60 years. The differential diagnosis of an adrenal mass is extensive and includes both secreting and nonsecreting neoplasms (Fig. 39-18). In patients with a history of malignancy, metastatic disease is the most likely cause of adrenal masses, particularly when bilateral (see Metastases to the Adrenal Gland, later). In those without a clear history of malignancy, at least 80% of incidentalomas will turn out to be nonfunctioning cortical adenomas or other benign lesions that do not require surgical management. Thus, in most patients, the most important aspect of management is to distinguish the subset of adrenal masses that are likely to have a clinical impact from the large proportion that will not.
B
Figure 39-17 Gross appearance of pheochromocytoma. A, Open
resection of a left para-aortic extra-adrenal pheochromocytoma (depicted in Fig. 39-16B) via an infracolic approach. The patients head is to the right. The tumor is being rotated medially by the surgeons hand to reveal the left ureter, indicated by forceps. B, Left adrenal pheochromocytoma. (A, Courtesy of Stan Sidhu, PhD.)
more likely to be multifocal. Succinate dehydrogenase B mutation carriers have high rates of extra-adrenal (abdominal or thoracic) pheochromocytomas and malignant disease, whereas succinate dehydrogenase D carriers tend to have multiple tumors and hormonally inactive paragangliomas of the head and neck. The lifetime penetrance of succinate dehydrogenase mutations is estimated to be greater than 75%.43 Genetic counseling and testing is encouraged for patients in whom pheochromocytoma is diagnosed before the age of 50.
Clinical Evaluation and Surgical Management
The workup for adrenal incidentaloma integrates hormonal evaluation with size criteria. The principles and methods of hormonal evaluation are discussed earlier in the tumor-specic sections and are generally applicable to incidentalomas. However, one conceptual difference
Malignant Pheochromocytoma Depending on the underlying genotype, anywhere from 2.5% to 40% of pheochromocytomas are malignant. Sur-
1022
Section VIII Endocrine
10% 5% 5% 5% 5% 9%
1%
60%
Nonfunctioning adenoma Pheochromocytoma Cortisol producing adenoma Aldosteronoma Adrenocortical carcinoma Adrenal cyst Ganglioneuroma Myelolipoma
Figure 39-18 Differential diagnosis of adrenal incidentaloma in patients without a history of malignancy. Approxi-
mate proportions of the various pathologies are shown.
is that the biochemical thresholds that prompt operative treatment are somewhat lower in patients with an initial radiographic manifestation (incidentalomas) than in those with an initial clinical manifestation. This results from the fact that tumor size, which correlates strongly with risk for malignancy, contributes an additive effect in favor of surgical management. Evaluation begins with history taking, with a focus on previous malignancy, hypertension, and symptoms of glucocorticoid or sex steroid excess. Biochemical investigations for hormonally active tumors are followed by consideration of size criteria (Fig. 39-19). In a general sense, surgery is recommended for hormonally active tumors and those that carry a signicant risk for malignancy. Adrenocortical carcinomas represent less than 2% of adrenal tumors measuring 4 cm or less and roughly 6% of those measuring 4 to 6 cm. Tumors larger than 6 cm carry a greater than 25% risk for malignancy. Because studies have consistently found that CT and MRI underestimate adrenal tumor size by approximately 20% (an effect that is exaggerated in smaller tumors), our practice is to remove all incidentalomas measuring 5 cm or greater and to strongly consider removal of those measuring 3 to 5 cm.46 Factors that need to be considered in surgical decision making for this latter group include suspicious imaging characteristics (heterogeneity, high attenuation, or irregular margins), the patients age and surgical risk, growth on interval imaging, and patient preference. If observation is chosen, patients undergo repeat imaging in 6 to 12 months given the fact that 5% to 25% of adrenal masses may increase in size. CT-guided ne-needle aspiration is rarely helpful in the evaluation of adrenal masses and may be hazardous. The diagnosis of primary adrenal malignancy cannot be reliably based on cytologic criteria alone. Therefore, the use of ne-needle aspiration is generally conned to patients with a history of extra-adrenal malignancy in whom the clinician seeks to establish the diagnosis of metastatic disease. In all cases, pheochromocytoma must be excluded before attempting such a procedure to avoid precipitating potentially fatal hypertensive crisis. As with the other disease processes that have been discussed, most adrenal incidentalomas can be removed laparoscopically, except for those displaying obvious
malignant features on imaging. No upper size limit to this approach has been established, and tumors measuring 15 cm have been successfully removed laparoscopically by experienced surgeons.
METASTASES TO THE ADRENAL GLAND Epidemiology and Clinical Features
The adrenal glands are common sites of metastasis because of their rich vascular supply. In fact, autopsy studies reveal that adrenal involvement eventually develops in approximately 25% of patients with carcinoma. In half these cases, the metastatic disease is bilateral. The primary cancers that most often spread to the adrenals are those of the lung, gastrointestinal tract, breast, kidney, pancreas, and skin (melanoma).47 Patients with isolated adrenal metastases represent a very small subset of the total. However, these individuals are of particular interest to the surgeon and oncologist because growing evidence indicates that resection of isolated adrenal metastases may improve survival. The principal determinant of survival in these patients is the disease-free interval. Patients in whom metachronous isolated adrenal metastases develop more than 6 months after the initial cancer have better survival rates than those with synchronous adrenal metastases.
Clinical Evaluation and Surgical Management
Evaluation of patients with isolated adrenal metastases must involve careful exclusion of extra-adrenal disease with CT or MRI (including the head in cases of breast cancer or melanoma and triphasic contrast-enhanced CT evaluation of the liver plus 3-mm slices through the lungs for gastrointestinal malignancies), as well as bone scan and PET when appropriate. Patients with isolated bilateral adrenal metastases (Fig. 39-20) must be evaluated for adrenal insufciency secondary to replacement of all normal adrenal tissue with tumor, which may occur in up to 30% of such patients. This is best done by measurement of morning cortisol and ACTH. Cortisol insufciency must be adequately treated before surgery to avoid perioperative adrenal crisis.
Chapter 39 The Adrenal Glands
1023
Adrenal incidentaloma
Hormonal evaluation
24-hr urine metanephrines and catecholamines
Low-dose (1 mg) dexamethasone suppression test
PAC/PRA if hypertensive
Functioning adrenal tumor?
Yes
No Size criteria, risk/benefit assessment <3 cm Consider tumor size 3-5 cm Consider case-specific factors: Suspicious imaging features Young patient Few surgical risk factors Interval tumor growth Patient preference No Yes >5 cm
Management
Interval CT in 6 months
Adrenalectomy
Figure 39-19 Algorithm for management of an adrenal incidentaloma. Adrenalectomy is recommended for all
patients with functional tumors. For nonfunctioning tumors, the risk for malignancy is assessed according to size. Tumors larger than 5 cm on CT carry a greater than 25% risk for malignancy and need to be removed. Those smaller than 3 cm can be safely observed. Case-specic factors must be considered for intermediate-sized tumors. PAC, plasma aldosterone concentration; PRA, plasma renin activity.
The great majority of adrenal metastases are well encapsulated and thus amenable to laparoscopic resection. Complete adrenal metastasectomy has yielded a mean survival of 20 to 30 months in most series,48 as compared with 12 months for patients with incomplete resection and 6 months for patients not undergoing surgical therapy.
TECHNICAL ASPECTS OF ADRENALECTOMY Choice of Operative Approach
In our practice, approximately 90% of adrenalectomies are performed laparoscopically. Laparoscopic adrenalectomy affords many advantages over conventional open surgery, including reduced length of hospitalization, reduced pain, decreased operative blood loss, and a lower rate of postoperative complications.49 Similar degrees of benet are observed with transabdominal and
posterior retroperitoneal laparoscopic approaches. Because of the wider operative eld and greater versatility afforded by the lateral transabdominal technique, it is our favored approach and is discussed in greater detail later. The lateral transabdominal approach can be used to handle very large tumors, and previous abdominal surgery does not alter the success rate signicantly when the procedure is performed by an experienced surgeon. The overall conversion rate to open adrenalectomy is less than 5% in large series. As discussed earlier, open adrenalectomy is performed for primary adrenal tumors demonstrating features suggestive of malignancy, such as large size (>8 cm), clinical feminization, hypersecretion of multiple steroid hormones, or any of the following imaging attributes: local/ vascular invasion, regional adenopathy, and metastases. For open adrenalectomy, we also prefer a transabdominal approach, which is performed via a subcostal incision as discussed later.
1024
Section VIII Endocrine
Figure 39-21 Patient positioning for left lateral transabdominal
laparoscopic adrenalectomy.
Figure 39-20 Isolated bilateral 7-cm adrenal metastases from
colorectal cancer causing adrenal insufciency. The patient had undergone previous right colectomy and right hepatectomy. Bilateral adrenal metastasectomy was performed laparoscopically.
surgeon is reliant on gravity to serve as a retractor in providing the necessary exposure. Having the patient securely xed to the table permits the often extreme positioning with respect to pitch (Trendelenburg/reverse Trendelenburg) and roll (tilting left/right) that is necessary during the operation.
Laparoscopic Lateral Transabdominal Adrenalectomy
Patient Preparation and Positioning Draw sheets and a full-length beanbag are placed on the operating table in advance. It is important that the table be capable of exion and have a kidney rest that can be elevated. The patient is initially positioned supine for induction of anesthesia and placement of a urinary catheter. Intermittent pneumatic compression devices are applied to the legs. Placement of an orogastric or nasogastric tube for gastric decompression is frequently helpful, particularly when treating left-sided lesions. The patient is then turned on the side (80-degree lateral decubitus position), with the side bearing the lesion facing upward (Fig. 39-21). At this point the patient is carefully positioned in the cephalocaudal dimension such that the 10th rib is directly over the breakpoint in the table. The table is exed and the beanbag rigidied in a position that supports the buttocks and back while leaving the umbilicus (an important surface landmark) exposed. Flexing the table and raising the kidney rest serve to widen the space between the costal margin and the iliac crest and to drop the iliac crest away from the plane of the laparoscopic instruments. Wide cloth tape is used to secure the patient to the table at the chest, hips, and lower extremities. Great care must taken to protect bony prominences and points of potential peripheral nerve compression in the extremities. The surgical preparation is carried from the nipple line to the pubis and from the umbilicus to the midline of the back. Careful positioning is essential for technical success in laparoscopic adrenalectomy. As discussed later, the
Technique: Left Adrenal Initial peritoneal access is achieved 2 cm inferior to the costal margin in the midclavicular line (Palmers point). This can be performed with either the Veress or Hasson technique. We generally use four 11-mm radially dilating (noncutting) trocars, which allows free exchange of the laparoscope, fan retractor, dissecting devices, and clip applier as needed. The ports are equally distributed along the costal margin, with the posterior port placed as far lateral/posterior as permitted by the position of the colon (Fig. 39-22). It is advisable to leave at least 5 cm (4 ngerbreadths) between each port to minimize external interference of the laparoscopic instruments. For tissue dissection, we use the hook monopolar cautery and an energy-based tissue sealing/dividing device. The lateral attachments of the spleen are taken down rst, with the goal of rotating the left upper quadrant viscera anteromedially. Splenic mobilization is continued until the greater curvature of stomach becomes visible at its apex, at which point the spleen and tail of the pancreas are allowed to fall anteriorly with rightward tilting of the table and gentle use of the fan retractor. In many patients, the splenic exure of the colon must be mobilized caudally by dividing the splenocolic ligament. We use an open book technique that involves developing the cleftlike plane just medial to the adrenal gland and lateral to the aorta (Fig. 39-23). The left-hand page of the book is composed of the spleen, tail of the pancreas, and greater curvature of the stomach. The right-hand page of the book consists of the kidney and adrenal tumor. The left crus of the diaphragm is a useful landmark that leads the surgeon to the left inferior phrenic vein. As mentioned in the anatomy section of this chapter, the left inferior phrenic vein courses along the medial aspect of
Chapter 39 The Adrenal Glands
1025
Pancreas Stomach Spleen
Left inferior phrenic vein Adrenal
Kidney
Figure 39-22 Port placement for right laparoscopic adrenalectomy. In this gure the patient is lying right-side up with the head toward the right. The marked line denotes the costal margin. Ports are placed approximately 2 cm inferior to the costal margin, spaced about 4 ngerbreadths apart.
Adrenal vein
A
Stomach Spleen
Colon
Left renal vein Pancreas Left inferior phrenic vein Adrenal
the left adrenal gland before joining with the left adrenal vein. By developing the cleft of the open book, moving in a superior-to-inferior direction, the adrenal vein is encountered at the inferomedial aspect of the adrenal gland. The small adrenal arteries that lie within this plane can be handled with energy-based coagulation. The left adrenal vein is carefully dissected out, clipped (leaving two clips on the patient side), and divided. The inferior tip of the left adrenal gland may extend quite low and approach the renal hilum within millimeters. However, because the left adrenal vein is rather long (2 cm), it is not generally necessary to expose the renal vasculature during left adrenalectomy. The adrenal gland is liberated by completing the dissection circumferentially and posteriorly and taking the specimen off the superior pole of the kidney and posterior abdominal wall. These attachments are deliberately divided last because they aid in suspending the adrenal gland on the lateral/superior wall of the operative eld, thereby providing exposure of the medial vascular plane during the critical initial portion of the procedure. The tumor is placed in a resilient catchment device, morcellated, and extracted. If noncutting trocars are used, only the skin need be closed.
Kidney
Colon
Adrenal vein
Figure 39-23 Technique of left laparoscopic adrenalectomy. The spleen and pancreatic tail have been mobilized and retracted anteromedially to expose the adrenal gland. The cleft of the open book is developed in a superior-to-inferior direction to identify the inferior phrenic vein and adrenal vein.
Technique: Right Adrenal Laparoscopic right adrenalectomy is, in some respects, a mirror image of the procedure just described. During right adrenalectomy, the left-hand page of the open book is made up of the kidney and adrenal tumor, and the right-hand page consists of the bare area of the liver (Fig. 39-24). To gain access to the appropriate plane, the right triangular ligament of the liver must rst be completely mobilized and the liver allowed to rotate anteromedially. On the right side, the colon usually lies well inferior to the operative eld. When developing the space between
the adrenal gland and inferior vena cava superiorly to inferiorly, the surgeon must be mindful of adrenal vein variants, as illustrated in the anatomy section of this chapter (see Fig. 39-3). The right adrenal vein is a potentially perilous structure to manage because it is short, wide, variable, and conuent with thin-walled, large capacitance vessels (the inferior vena cava in >80% of cases, followed by the renal vein and uncommonly the right hepatic vein) that can bleed briskly if directly injured (e.g., by cautery), lacerated from undue traction on adjacent structures, or sheared by clips. A signicant second
1026
Section VIII Endocrine
Figure 39-24 Technique of right laparoscopic adrenalectomy. The liver has been mobilized and retracted medially to expose the adrenal gland and inferior vena cava. The space just medial to the adrenal gland is developed to identify the adrenal vasculature.
adrenal vein may be found in up to 10% of patients. By methodically dissecting one layer at a time and moving in a superior-to-inferior direction, all potential adrenal vein variants can be encountered in controlled fashion. The adrenal vein must be dissected out delicately, denitively ligated (again usually with two clips on the patient side), and then divided. Loss of control of the adrenal vein stump needs to be avoided; if it occurs, conversion to an open procedure may be necessary. Of note, the junction of the inferior vena cava and right renal vein is frequently difcult to identify. In vivo, the transition is a gradual curve rather than the 90-degree takeoff depicted in anatomy texts. Therefore, it cannot be used as a reliable anatomic landmark for identication of the adrenal vein.
Figure 39-25 Patient positioning for open right adrenalectomy.
Patients who undergo laparoscopic adrenalectomy recover rapidly. Most patients, including approximately half of those treated for pheochromocytoma, are able to leave the hospital on the rst postoperative day. In the treatment of adrenal tumors, successful outcomes hinge on excellent perioperative medical management as much as technical skill, particularly in cases of pheochromocytoma and Cushings syndrome. These considerations are discussed earlier in the disease-specic sections.
Open Anterior Transabdominal Adrenalectomy
Patient Preparation and Positioning Neuraxial blockade (use of an epidural catheter) is routinely used for intraoperative and postoperative anesthetic/analgesic management. The patient is positioned supine, with the ipsilateral side slightly elevated on a bolster (Fig. 39-25). A urinary catheter, orogastric or nasogastric tube, and intermittent pneumatic compression devices are placed. The surgical preparation is carried from the nipple line to the pubis and down to the table on either side. Technique: Left Adrenal We prefer to use a subcostal incision, which may be extended across the midline (chevron) with or without a vertical upper midline extension to achieve wide exposure. The left adrenal can be exposed by entering the lesser sac through the gastrocolic ligament and incising
Complications and Postoperative Care Potential technical complications include venous hemorrhage and bleeding from solid organ capsular injuries. Small amounts of bleeding can often be managed by coagulation or direct pressure with rolled Kittner gauze. Hollow viscus injuries are uncommon but may be associated with procedures performed in patients who have previously undergone major abdominal surgery. Pancreatic injuries and stulas have been reported with leftsided procedures but are rare complications, as are port site hernias and port site metastases in cases of malignancy. Patients undergoing laparoscopic adrenalectomy for Cushings syndrome are at risk for surgical site infections, including port site infections in 5% to 10% of cases and, rarely, subphrenic abscesses requiring catheter drainage, as a result of their catabolic and immunosuppressed state.
Chapter 39 The Adrenal Glands
1027
Gallbladder Liver
Right adrenal vein
Pancreas Duodenum Inferior vena cava Transverse colon
Figure 39-26 Open right adrenalectomy. The right lobe of the liver and the hepatic exure of the colon have been completely mobilized. The retroperitoneum is entered and the duodenum and head of the pancreas reected medially (Kocher maneuver) to expose the adrenal gland and inferior vena cava.
the retroperitoneum inferior to the tail of the pancreas or by rotating the spleen, pancreatic tail, and stomach anteromedially as described in the section on laparoscopic adrenalectomy. The latter approach is used in our practice. The splenic exure of the colon is mobilized inferiorly and the plane medial to the adrenal gland developed. The adrenal vein is isolated, tied in continuity, and divided. The small adrenal arteries can be ligated or electrocoagulated and the specimen removed after circumferential dissection is completed.
Technique: Right Adrenal Open right adrenalectomy begins with complete mobilization of the right lobe of the liver, including the lateral attachments and the falciform ligament. The adrenal can be exposed by rotating the liver medially or, more commonly, retracting the inferoposterior segments cephalad with long, padded retractors (liver, renal vein, Deaver, or Harrington types). The retroperitoneum is entered by
performing a Kocher maneuver (Fig. 39-26) and the inferior vena cava exposed by medial reection of the duodenum. The plane between the adrenal gland and inferior vena cava is developed rst. Vascular structures, which may be numerous in highly angiogenic tumors, are ligated sequentially. The adrenal vein is isolated, securely tied, and divided. Loss of control of the adrenal vein stump may be managed with the application of a side-biting (Satinsky) vascular clamp. As discussed earlier, open adrenalectomy is generally performed in cases of suspected or known malignancy. Locally invasive right-sided adrenal tumors can be challenging to manage given their frequent invasion of adjacent venous structures (Fig. 39-27). It is our practice to involve an experienced vascular or liver surgeon in the management of tumors with extensive venous invasion. Locally invaded organs, most commonly the kidney, are resected en bloc with the primary mass. Complete radical resection is a critical determinant of survival in patients with malignant adrenal
1028
Section VIII Endocrine
Figure 39-27 Open resection of a right adrenocortical carcinoma invading the inferior vena cava. The patients head is to the left. The liver (white arrowhead) is retracted cephalad. The white arrow indicates the tumor and the black arrow indicates the inferior vena cava, which is encircled with vessel loops.
tumors; in some cases this can be achieved only if immediate venous reconstruction is performed (Fig. 39-28).
Complications and Postoperative Care Technical complications of open adrenalectomy include venous hemorrhage, tumor embolization in patients with intravascular tumor extension, and solid organ injury. Postoperative complications are similar to those associated with other major abdominal procedures. Most patients experience return of bowel function within 3 to 4 days and are able to leave the hospital on postoperative day 5 to 7.
Selected References
Axelrod L: Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin North Am 32:367-383, 2003.
A thorough review of existing nonsystematic studies on steroid use that provides clear guidelines and the rationale behind them.
B
Figure 39-28 Open resection of a right adrenocortical carcinoma
Gifford RW Jr, Kvale WF, Maher FT, et al: Clinical features, diagnosis and treatment of pheochromocytoma: A review of 76 cases. Mayo Clin Proc 39:281-302, 1964.
A landmark account of the biochemical, pharmacologic, and physiologic advances that allowed collaborators at the Mayo Clinic to treat 76 patients with pheochromocytoma while experiencing only one death.
necessitating vascular reconstruction. A, The infrahepatic inferior vena cava has been replaced with a polytetrauoroethylene graft. The liver can be seen superiorly and the colon inferiorly. B, Ex vivo specimen consisting of the right adrenal tumor with the kidney resected en bloc. The renal vein is indicated by the arrow. Forceps have been placed through the resected segment of inferior vena cava.
A critical analysis of biochemical test operating characteristics in the diagnosis of pheochromocytoma.
Grumbach MM, Biller BM, Braunstein GD, et al: Management of the clinically inapparent adrenal mass (incidentaloma). Ann Intern Med 138:424-429, 2003.
A summary statement from the National Institutes of Health Consensus Development Program with recommendations on incidentaloma workup and indications for surgery.
Lindholm J, Juul S, Jorgensen JO, et al: Incidence and late prognosis of Cushings syndrome: A population-based study. J Clin Endocrinol Metab 86:117-123, 2001.
Documents the excess mortality associated with Cushings syndrome.
Milliez P, Girerd X, Plouin PF, et al: Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45:1243-1248, 2005.
A compelling report on the adverse effects of primary aldosteronism in which affected patients are compared with controls matched for systolic blood pressure.
Kudva YC, Sawka AM, Young WF Jr: Clinical review 164: The laboratory diagnosis of adrenal pheochromocytoma: The Mayo Clinic experience. J Clin Endocrinol Metab 88:4533-4539, 2003.
Chapter 39 The Adrenal Glands
1029
Minneci PC, Deans KJ, Banks SM, et al: Meta-analysis: The effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med 141:47-56, 2004.
A useful analysis of various steroid regimens in the treatment of critically ill patients with relative adrenal insufciency.
Neumann HP, Bausch B, McWhinney SR, et al: Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 346:1459-1466, 2002.
A landmark report documenting a surprisingly high rate of familial pheochromocytoma and calling attention to the signicance of succinate dehydrogenase mutations.
Ng L, Libertino JM: Adrenocortical carcinoma: Diagnosis, evaluation and treatment. J Urol 169:5-11, 2003.
A multi-institutional descriptive report on this rare disease.
Nobel Lectures, Physiology or Medicine 1942-1962. Amsterdam, Elsevier, 1964.
An account of the clinical discoveries and advances in organic chemistry that led to identication, isolation, and articial synthesis of adrenal cortical hormones. A full transcript can be found at http://nobelprize. org.
Nobel Lectures, Physiology or Medicine 1971-1980. Amsterdam, Elsevier, 1992.
Documents the formidable challenges surmounted in the identication of peptide hormones, found in such minute concentrations, and development of the radioimmunoassay necessary for their detection. A full transcript can be found at http://nobelprize.org.
Plouin PF, Duclos JM, Soppelsa F, et al: Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: Analysis of 165 operations at a single center. J Clin Endocrinol Metab 86:1480-1486, 2001.
One of the largest single-institution series on surgical management of pheochromocytoma documents the evolution in clinical care over more than 2 decades.
Welbourn RB: Early surgical history of phaeochromocytoma. Br J Surg 74:594-596, 1987.
Describes the initial achievements of American and European surgeons in the successful treatment of pheochromocytoma.
Young WF, Stanson AW, Thompson GB, et al: Role for adrenal venous sampling in primary aldosteronism. Surgery 136:12271235, 2004.
A large single-institution series documenting the unreliability of CT alone in localizing aldosteronomas. The discussion that follows the main text is particularly informative.
References
1. Nobel Lectures, Physiology or Medicine 1942-1962. Amsterdam, Elsevier, 1964. 2. Nobel Lectures, Physiology or Medicine 1971-1980. Amsterdam, Elsevier, 1992. 3. Mihai R, Farndon JR: Surgical embryology and anatomy of the adrenal glands. In Clark OH, Duh QY, Kebebew E (eds): Textbook of Endocrine Surgery, 2nd ed. Philadelphia, Elsevier Saunders, 2005. 4. MacGillivray DC, Khwaja K, Shickman SJ: Conuence of the right adrenal vein with the accessory right hepatic veins. A potential hazard in laparoscopic right adrenalectomy. Surg Endosc 10:1095-1096, 1996.
5. Lack EE: Tumors of the adrenal gland and extra-adrenal paraganglia. In Rosai J (ed): Atlas of Tumor Pathology, vol 19. Washington, DC, Armed Forced Institute of Pathology, 1997. 6. Stocco DM: StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol 63:193-213, 2001. 7. Sapolsky RM, Romero LM, Munck AU: How do glucocorticoids inuence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55-89, 2000. 8. Lenders JW, Pacak K, Walther MM, et al: Biochemical diagnosis of pheochromocytoma: Which test is best? JAMA 287:1427-1434, 2002. 9. Speiser PW, White PC: Congenital adrenal hyperplasia. N Engl J Med 349:776-788, 2003. 10. Nieman LK, Chanco Turner ML: Addisons disease. Clin Dermatol 24:276-280, 2006. 11. Ten S, New M, Maclaren N: Clinical review 130: Addisons disease 2001. J Clin Endocrinol Metab 86:2909-2922, 2001. 12. Shen WT, Kebebew E, Clark OH, et al: Selective use of steroid replacement after adrenalectomy: Lessons from 331 consecutive cases. Arch Surg 141:771-774, discussion 774776, 2006. 13. Minneci PC, Deans KJ, Banks SM, et al: Meta-analysis: The effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med 141:47-56, 2004. 14. de Herder WW, van der Lely AJ: Addisonian crisis and relative adrenal failure. Rev Endocr Metab Disord 4:143-147, 2003. 15. Dorin RI, Qualls CR, Crapo LM: Diagnosis of adrenal insufciency. Ann Intern Med 139:194-204, 2003. 16. Axelrod L: Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin North Am 32:367-383, 2003. 17. Kaplan NM: The current epidemic of primary aldosteronism: Causes and consequences. J Hypertens 22:863-869, 2004. 18. Mulatero P, Stowasser M, Loh KC, et al: Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from ve continents. J Clin Endocrinol Metab 89:1045-1050, 2004. 19. Milliez P, Girerd X, Plouin PF, et al: Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45:1243-1248, 2005. 20. Doi SA, Abalkhail S, Al-Qudhaiby MM, et al: Optimal use and interpretation of the aldosterone renin ratio to detect aldosterone excess in hypertension. J Hum Hypertens 20:482-489, 2006. 21. Young WF, Stanson AW, Thompson GB, et al: Role for adrenal venous sampling in primary aldosteronism. Surgery 136:1227-1235, 2004. 22. Harvey A, Kline G, Pasieka JL: Adrenal venous sampling in primary hyperaldosteronism: Comparison of radiological with biochemical success and the clinical decision making with less than ideal testing. Surgery 140:847-853, discussion 853-855, 2006. 23. Lal G, Duh QY: Laparoscopic adrenalectomyindications and technique. Surg Oncol 12:105-123, 2003. 24. Tan YY, Ogilvie JB, Triponez F, et al: Selective use of adrenal venous sampling in the lateralization of aldosteroneproducing adenomas. World J Surg 30:879-885, discussion 886-877, 2006. 25. Lindholm J, Juul S, Jorgensen JO, et al: Incidence and late prognosis of Cushings syndrome: A population-based study. J Clin Endocrinol Metab 86:117-123, 2001.
1030
Section VIII Endocrine
26. Papanicolaou DA, Mullen N, Kyrou I, et al: Nighttime salivary cortisol: A useful test for the diagnosis of Cushings syndrome. J Clin Endocrinol Metab 87:4515-4521, 2002. 27. Rockall AG, Babar SA, Sohaib SA, et al: CT and MR imaging of the adrenal glands in ACTH-independent Cushing syndrome. Radiographics 24:435-452, 2004. 28. Newell-Price J, Bertagna X, Grossman AB, et al: Cushings syndrome. Lancet 367:1605-1617, 2006. 29. Esposito F, Dusick JR, Cohan P, et al: Clinical review: Early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushings disease. J Clin Endocrinol Metab 91:7-13, 2006. 30. Findling JW, Raff H: Cushings syndrome: Important issues in diagnosis and management. J Clin Endocrinol Metab 91:3746-3753, 2006. 31. Tauchmanova L, Rossi R, Biondi B, et al: Patients with subclinical Cushings syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab 87:4872-4878, 2002. 32. Ng L, Libertino JM: Adrenocortical carcinoma: Diagnosis, evaluation and treatment. J Urol 169:5-11, 2003. 33. Icard P, Goudet P, Charpenay C, et al: Adrenocortical carcinomas: Surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg 25:891-897, 2001. 34. Dackiw AP, Lee JE, Gagel RF, et al: Adrenal cortical carcinoma. World J Surg 25:914-926, 2001. 35. Welbourn RB: Early surgical history of phaeochromocytoma. Br J Surg 74:594-596, 1987. 36. Pacak K, Linehan WM, Eisenhofer G, et al: Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med 134:315-329, 2001. 37. Dluhy RG: Pheochromocytomadeath of an axiom. N Engl J Med 346:1486-1488, 2002. 38. Kudva YC, Sawka AM, Young WF Jr: Clinical review 164: The laboratory diagnosis of adrenal pheochromocytoma: The Mayo Clinic experience. J Clin Endocrinol Metab 88:4533-4539, 2003.
39. Ilias I, Pacak K: Current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. J Clin Endocrinol Metab 89:479-491, 2004. 40. Gifford RW Jr, Kvale WF, Maher FT, et al: Clinical features, diagnosis and treatment of pheochromocytoma: A review of 76 cases. Mayo Clin Proc 39:281-302, 1964. 41. Plouin PF, Duclos JM, Soppelsa F, et al: Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: Analysis of 165 operations at a single center. J Clin Endocrinol Metab 86:1480-1486, 2001. 42. Neumann HP, Bausch B, McWhinney SR, et al: Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 346:1459-1466, 2002. 43. Benn DE, Gimenez-Roqueplo AP, Reilly JR, et al: Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab 91:827-836, 2006. 44. Edstrom Elder E, Hjelm Skog AL, Hoog A, et al: The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol 29:278-283, 2003. 45. Grumbach MM, Biller BM, Braunstein GD, et al: Management of the clinically inapparent adrenal mass (incidentaloma). Ann Intern Med 138:424-429, 2003. 46. Sturgeon C, Kebebew E: Laparoscopic adrenalectomy for malignancy. Surg Clin North Am 84:755-774, 2004. 47. Lam KY, Lo CY: Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 56:95-101, 2002. 48. Sebag F, Calzolari F, Harding J, et al: Isolated adrenal metastasis: The role of laparoscopic surgery. World J Surg 30:888892, 2006. 49. Shen WT, Kebebew E, Clark OH, et al: Reasons for conversion from laparoscopic to open or hand-assisted adrenalectomy: Review of 261 laparoscopic adrenalectomies from 1993 to 2003. World J Surg 28:1176-1179, 2004.
Вам также может понравиться
- Carol Hanisch - The Personal Is PoliticalДокумент5 страницCarol Hanisch - The Personal Is Politicalconradofalbo100% (1)
- Bacterial MeningitisДокумент125 страницBacterial MeningitisTessa Balboa-Tuason90% (10)
- Tumours of The Adrenal GlandДокумент34 страницыTumours of The Adrenal GlandSonam JoshiОценок пока нет
- Approach To The Patient With An Adnexal Mass - UpToDateДокумент31 страницаApproach To The Patient With An Adnexal Mass - UpToDateRamackОценок пока нет
- The Integumentary System Development: Biene, Ellen Angelic Flores, Andrie BonДокумент29 страницThe Integumentary System Development: Biene, Ellen Angelic Flores, Andrie BonMu Lok100% (3)
- G.I. GURDJIEFF - INDEX TO BEELZEBUBS TALES TO HIS GRANDSON - Arkana 99 EDITIONДокумент68 страницG.I. GURDJIEFF - INDEX TO BEELZEBUBS TALES TO HIS GRANDSON - Arkana 99 EDITIONpetemurdoch5265100% (7)
- Suprarenal (Adrenal) Gland: Dr. R. SanthakumarДокумент33 страницыSuprarenal (Adrenal) Gland: Dr. R. SanthakumardrsubanОценок пока нет
- Journal Reading RSP Conn Syndrome Aulia PDFДокумент33 страницыJournal Reading RSP Conn Syndrome Aulia PDFAhmad Nur AuliaОценок пока нет
- Acute PancreatitisДокумент46 страницAcute PancreatitisLew NianОценок пока нет
- CSFДокумент40 страницCSFDrNaveen Singh Rajpurohit KaduОценок пока нет
- Dr. Tjatur Winarsanto SPPDДокумент37 страницDr. Tjatur Winarsanto SPPDEndah Risky GustiyantiОценок пока нет
- Chronic Myeloproliferative Disorders: Matthew G. Yap, MD, FPCP, FPSHBT, FpsmoДокумент42 страницыChronic Myeloproliferative Disorders: Matthew G. Yap, MD, FPCP, FPSHBT, FpsmoDon RaulОценок пока нет
- Tumours of The Central Nervous System: FM Brett MD., FrcpathДокумент57 страницTumours of The Central Nervous System: FM Brett MD., FrcpathDrGasnasОценок пока нет
- Carcinoma of The Breast - Bailey & LoveДокумент5 страницCarcinoma of The Breast - Bailey & LoveKeyshia Yazid100% (1)
- The Liver: Xu Bing, M.D. Associate ProfessorДокумент71 страницаThe Liver: Xu Bing, M.D. Associate Professorapi-19916399Оценок пока нет
- Extra Aksial Brain TumorsДокумент15 страницExtra Aksial Brain TumorsPPDSNeuroUnsri RSMH0% (1)
- Cerebrospinal CSFДокумент31 страницаCerebrospinal CSFRashid MohamedОценок пока нет
- 01 - Signs and Symptoms of Git DisordersДокумент51 страница01 - Signs and Symptoms of Git DisordersRere AnugrahОценок пока нет
- Suprarenal GlandДокумент19 страницSuprarenal GlandKay BristolОценок пока нет
- Sickle Cell Disease: Everything You Ever Wanted To KnowДокумент23 страницыSickle Cell Disease: Everything You Ever Wanted To KnowAnastasiafynn100% (1)
- Seminar On Cellulitis: Ms. J - Sheela Rajakumari MSC Nursing 1 Year MMM College of NursingДокумент20 страницSeminar On Cellulitis: Ms. J - Sheela Rajakumari MSC Nursing 1 Year MMM College of NursingSheela Raja Kumari100% (1)
- 4 Circulation Disorders PDFДокумент69 страниц4 Circulation Disorders PDFSetiawan SukmadjaОценок пока нет
- Differential Diagnosis of The Adnexal Mass 2020Документ38 страницDifferential Diagnosis of The Adnexal Mass 2020Sonia MVОценок пока нет
- Adrenal DisordersДокумент48 страницAdrenal DisordersMubeenUrRehmanОценок пока нет
- Case 1 - Sickle Cell Disease - PPSXДокумент44 страницыCase 1 - Sickle Cell Disease - PPSXNICHOLAS KAUMBA100% (1)
- Presentation JaundiceДокумент49 страницPresentation JaundiceVinoth KumarОценок пока нет
- ArteriosclerosisДокумент8 страницArteriosclerosisDr. Bushra SumraОценок пока нет
- Bio StatisticsДокумент122 страницыBio StatisticsSweta SaravananОценок пока нет
- Pathology ChartsДокумент21 страницаPathology Chartspadma maliniОценок пока нет
- Pancreatic MassДокумент124 страницыPancreatic MassKMОценок пока нет
- Hepato-Biliary System 26.4.2016Документ59 страницHepato-Biliary System 26.4.2016S B SayedОценок пока нет
- THROMBOSISДокумент18 страницTHROMBOSISShruti Verma100% (1)
- Lower Urinary Tract Symptoms (LUTS)Документ26 страницLower Urinary Tract Symptoms (LUTS)Ayi Abdul BasithОценок пока нет
- Diagnostic Cardiac EnzymesДокумент27 страницDiagnostic Cardiac Enzymesد.لطفي دحمانОценок пока нет
- MenorrhagiaДокумент4 страницыMenorrhagiaFlloyd_Martin__1259Оценок пока нет
- Anatomy of The Bony PelvisДокумент16 страницAnatomy of The Bony Pelvismichael0202Оценок пока нет
- Acute Kidney InjuryДокумент37 страницAcute Kidney InjuryLani BuenaventuraОценок пока нет
- Pathophysiology 2Документ92 страницыPathophysiology 2Princess AgarwalОценок пока нет
- Renal PathologyДокумент28 страницRenal PathologyApril Deveras JudillaОценок пока нет
- Presented by T. V. L. Sahithi Ist Year PG Dept of PeriodonticsДокумент49 страницPresented by T. V. L. Sahithi Ist Year PG Dept of Periodonticslakshmi sahithi natakalaОценок пока нет
- Cholesistitis (DR - Prema Hapsari, SPPD)Документ28 страницCholesistitis (DR - Prema Hapsari, SPPD)Niniek Iin F100% (1)
- Paper Presentation On Trichobezoars A Hairy Cause of Intestinal ObstructionДокумент14 страницPaper Presentation On Trichobezoars A Hairy Cause of Intestinal ObstructionAimanОценок пока нет
- Varian Cysts: The Lebanese Society of Obstetrics and GynecologyДокумент4 страницыVarian Cysts: The Lebanese Society of Obstetrics and GynecologyAde Gustina SiahaanОценок пока нет
- Physiology of The Gastrointestinal Tract (Git)Документ98 страницPhysiology of The Gastrointestinal Tract (Git)jballungayОценок пока нет
- Kidney: Biochemical Tests For Assessing Renal FunctionsДокумент56 страницKidney: Biochemical Tests For Assessing Renal FunctionsPaulina Paskeviciute100% (1)
- Head Injury 1Документ33 страницыHead Injury 1drvishal bhattОценок пока нет
- Cough, Dyspnea and HemoptysisДокумент34 страницыCough, Dyspnea and HemoptysisPooja ShashidharanОценок пока нет
- 10 EdemaДокумент23 страницы10 EdemaTalmaciu AmyОценок пока нет
- Abdominal Wall Extraskeletal Ewing Sarcoma - Case ReportДокумент3 страницыAbdominal Wall Extraskeletal Ewing Sarcoma - Case ReportInternational Organization of Scientific Research (IOSR)Оценок пока нет
- ShockДокумент21 страницаShockMin-Joo Esther ParkОценок пока нет
- Compartment Syndrome: Sebelas Maret UniversityДокумент8 страницCompartment Syndrome: Sebelas Maret Universitykhrisna satyaksaОценок пока нет
- PPTДокумент61 страницаPPTHendra Devandra100% (1)
- Kidney AnatomyДокумент2 страницыKidney Anatomyameerabest100% (1)
- Adequacy Criteria: ExceptionsДокумент3 страницыAdequacy Criteria: ExceptionsPranayОценок пока нет
- Disorders of Parathyroid GlandsДокумент52 страницыDisorders of Parathyroid GlandsDr. Akash GuptaОценок пока нет
- Anorectal Suppuration 9.7.11!1!1Документ50 страницAnorectal Suppuration 9.7.11!1!1Shantu Shirurmath100% (2)
- 1st Lecture On The Histology of Female Reproductive System by Dr. RoomiДокумент17 страниц1st Lecture On The Histology of Female Reproductive System by Dr. RoomiMudassar RoomiОценок пока нет
- Pancreatic CancerДокумент25 страницPancreatic CancerAatir JavaidОценок пока нет
- Nephrotic SyndromeДокумент24 страницыNephrotic SyndromeJawad SaleemОценок пока нет
- Pathology of Parathyrid Gland FinalДокумент63 страницыPathology of Parathyrid Gland FinalGurpreet Kaur SagooОценок пока нет
- A. Cardio: Anatomy & Physiology - A. AnatomyДокумент43 страницыA. Cardio: Anatomy & Physiology - A. AnatomyGloryJaneОценок пока нет
- SepsisДокумент33 страницыSepsisv_vijayakanth7656Оценок пока нет
- HematuriaДокумент42 страницыHematuriaWasim R. IssaОценок пока нет
- A Simple Guide to Adrenal Cancer, Diagnosis, Treatment and Related ConditionsОт EverandA Simple Guide to Adrenal Cancer, Diagnosis, Treatment and Related ConditionsОценок пока нет
- A Systems Approach To Improving Maternal Health in The PhilippinesДокумент7 страницA Systems Approach To Improving Maternal Health in The Philippinesashgee1Оценок пока нет
- NIH Public Access: Author ManuscriptДокумент16 страницNIH Public Access: Author Manuscriptashgee1Оценок пока нет
- Congenital Adrenal HyperplasiaДокумент13 страницCongenital Adrenal Hyperplasiaashgee1Оценок пока нет
- Costs of Hospital Care For Hypertension in The PhilippinesДокумент9 страницCosts of Hospital Care For Hypertension in The PhilippinesLiz CarreonОценок пока нет
- Clinical Transplantation of A Tissue-Engineered AirwayДокумент8 страницClinical Transplantation of A Tissue-Engineered Airwayashgee1Оценок пока нет
- Quantifying The Impoverishing Effects of Purchasing Medicines: A Cross-Country Comparison of The Affordability of Medicines in The Developing WorldДокумент8 страницQuantifying The Impoverishing Effects of Purchasing Medicines: A Cross-Country Comparison of The Affordability of Medicines in The Developing Worldashgee1Оценок пока нет
- Airway Xplant 2009feb Macchiarini Lancet CommentДокумент2 страницыAirway Xplant 2009feb Macchiarini Lancet Commentashgee1Оценок пока нет
- Congenital Adrenal HyperplasiaДокумент13 страницCongenital Adrenal Hyperplasiaashgee1Оценок пока нет
- Rheology of Leukocytes, Leukocyte Suspensions and Blood in LeukemiaДокумент9 страницRheology of Leukocytes, Leukocyte Suspensions and Blood in Leukemiaashgee1Оценок пока нет
- Diffusion Across A Sheep Red Blood Cell MembraneДокумент26 страницDiffusion Across A Sheep Red Blood Cell MembraneGelo JosonОценок пока нет
- THC Detection WindowДокумент16 страницTHC Detection Windowashgee1Оценок пока нет
- OSMOTIC STUDIES OF AMPHIBIAN EGGS. I. PRELIMINARY SURVEY OF VOLUME CHANGES by ORLANDO DE LUQUE AND F. R. HUNTER'Документ10 страницOSMOTIC STUDIES OF AMPHIBIAN EGGS. I. PRELIMINARY SURVEY OF VOLUME CHANGES by ORLANDO DE LUQUE AND F. R. HUNTER'ashgee1Оценок пока нет
- A Simple Membrane Osmometer System & Experiments That Quantitatively Measure Osmotic PressureДокумент9 страницA Simple Membrane Osmometer System & Experiments That Quantitatively Measure Osmotic Pressureashgee1Оценок пока нет
- Hazards of Smoke and Toxic Gases Produced in Urban FiresДокумент84 страницыHazards of Smoke and Toxic Gases Produced in Urban Firesashgee1Оценок пока нет
- A Proposed Monitoring SystemДокумент12 страницA Proposed Monitoring Systemashgee1Оценок пока нет
- JAMA 2000 Continuing Medical Education 943 4Документ2 страницыJAMA 2000 Continuing Medical Education 943 4ashgee1Оценок пока нет
- Contingency Planning: Business Impact AnalysisДокумент3 страницыContingency Planning: Business Impact Analysisashgee1Оценок пока нет
- Shell Scenarios Explorers - GuideДокумент98 страницShell Scenarios Explorers - GuidedaddotОценок пока нет
- A Hierarchical Model For Decentralized Fighting of Large Scale Urban FiresДокумент8 страницA Hierarchical Model For Decentralized Fighting of Large Scale Urban Firesashgee1Оценок пока нет
- Lessons For Health Care Reform From The Less Developed World: The Case of The PhilippinesДокумент7 страницLessons For Health Care Reform From The Less Developed World: The Case of The Philippinesashgee1Оценок пока нет
- The Health and Cost Impact of Care Delay and The Experimental Impact of Insurance On Reducing DelaysДокумент6 страницThe Health and Cost Impact of Care Delay and The Experimental Impact of Insurance On Reducing Delaysashgee1Оценок пока нет
- SC Office Directory: The Supreme Court TodayДокумент2 страницыSC Office Directory: The Supreme Court TodayAlex M TabuacОценок пока нет
- SC Office Directory: The Supreme Court TodayДокумент2 страницыSC Office Directory: The Supreme Court TodayAlex M TabuacОценок пока нет
- Class Activity 1Документ2 страницыClass Activity 1ashgee1Оценок пока нет
- New IRS Tax Tables February 2009Документ97 страницNew IRS Tax Tables February 2009Wayne Schulz88% (8)
- The Hukbalahap InsurrectionДокумент76 страницThe Hukbalahap Insurrectionashgee1Оценок пока нет
- The Hukbalahap MovementДокумент9 страницThe Hukbalahap Movementashgee1Оценок пока нет
- Liver DiseasesДокумент335 страницLiver DiseasesdipakrussiaОценок пока нет
- Ausarbeitung Immunologie A Korrigierte Fassung WS2019Документ185 страницAusarbeitung Immunologie A Korrigierte Fassung WS2019Adrienn MarkeszОценок пока нет
- LiverДокумент29 страницLiverHasan AsdiОценок пока нет
- WORKSHEET 3 Lymphocyte ActivationДокумент5 страницWORKSHEET 3 Lymphocyte ActivationNeha ChoudharyОценок пока нет
- Nervous System NotesДокумент21 страницаNervous System NotesLasalle BenavidesОценок пока нет
- Liver CancerДокумент1 страницаLiver CancerTarantado67% (3)
- Endocrine System DiseasesДокумент8 страницEndocrine System DiseasesPrincess Xzmae Ramirez100% (1)
- The Brain, PT 2 - Neuroscience and BehaviorДокумент2 страницыThe Brain, PT 2 - Neuroscience and BehaviorprtemnОценок пока нет
- Hap - Multiple Choice QuestionsДокумент11 страницHap - Multiple Choice QuestionsAnitha Mary DambaleОценок пока нет
- Clark Kent Manzano. Activity 1 What Makes Up An OrganismДокумент7 страницClark Kent Manzano. Activity 1 What Makes Up An OrganismMaria Christina Manzano100% (2)
- Homeostatic MechanismsДокумент5 страницHomeostatic MechanismsMonkey LoverОценок пока нет
- Intubation - Gastrointestinal TractДокумент2 страницыIntubation - Gastrointestinal TractDominion OgochukwuОценок пока нет
- Divine Intervention Step 2CK Podcasts Notes - Read Only File - Docx (Dragged) 6 (Dragged) 2Документ1 страницаDivine Intervention Step 2CK Podcasts Notes - Read Only File - Docx (Dragged) 6 (Dragged) 2winston1234Оценок пока нет
- Nephritic SyndromeДокумент23 страницыNephritic SyndromeLateefah TalalОценок пока нет
- Urinary Tract Infection (UTI)Документ6 страницUrinary Tract Infection (UTI)Anonymous iG0DCOfОценок пока нет
- SARIДокумент138 страницSARIohalu781391Оценок пока нет
- Physiology of The Cardiovascular System-CVSДокумент56 страницPhysiology of The Cardiovascular System-CVSAmanuel MaruОценок пока нет
- DR Ananta Thyroid SlideДокумент73 страницыDR Ananta Thyroid SlideRoshan Kumar PanditОценок пока нет
- Quiz For Grade 10Документ2 страницыQuiz For Grade 10Ralph Rexor Macarayan BantuganОценок пока нет
- Human Physiology-McqДокумент2 страницыHuman Physiology-Mcqaryan kothambiaОценок пока нет
- Abnormalities of PuerperiumДокумент16 страницAbnormalities of PuerperiumUjjawalShriwastavОценок пока нет
- 4mm Frog EmbryoДокумент3 страницы4mm Frog EmbryoIvy CruzОценок пока нет
- DLL - Science 4 - Q2 - W1Документ10 страницDLL - Science 4 - Q2 - W1Honeylyn CataytayОценок пока нет
- Pendahuluan Mata Kuliah FitoterapiДокумент16 страницPendahuluan Mata Kuliah FitoterapiQhoissul Saufus salfwaОценок пока нет
- Pathophysiology of Hypertension in Chronic Kidney DiseaseДокумент7 страницPathophysiology of Hypertension in Chronic Kidney DiseaseRifka WidianingrumОценок пока нет
- Science 9Документ29 страницScience 9HanessyОценок пока нет
- Gabr, Taha, Lab 15, Period 4Документ5 страницGabr, Taha, Lab 15, Period 4TAHA GABRОценок пока нет