Академический Документы
Профессиональный Документы
Культура Документы
Vidal Et Al BIOL PSY 03
Загружено:
melagkoliaИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Vidal Et Al BIOL PSY 03
Загружено:
melagkoliaАвторское право:
Доступные форматы
Biological Psychology 64 (2003) 265282
Error negativity on correct trials: a reexamination of available data
F. Vidal a,b, , B. Burle b , M. Bonnet b , J. Grapperon c , T. Hasbroucq a,b
a
Institut de Mdecine Navale du Service de Sant des Armes, BP 610, 83800 , Toulon Naval, France b Laboratoire de Neurobiologie de la Cognition, Centre National de la Recherche Scientique and Universit de Provence, Marseille, France c Laboratoire dExplorations Fonctionnelles du systme nerveux, H pital dInstruction des Armes, o Toulon, France Received 29 November 2001; accepted 31 January 2003
Abstract The error negativity, an EEG wave observed when subjects commit an error in a choice reaction time (RT) task, is often considered as a sign of error detection. Recently, reports of Ne-like waves on correct responses did challenge this interpretation. It has been proposed, however, that these Ne-like waves result either from an artifactual contamination of response-locked activities by stimulus-locked ones, or from an implicit monitoring of the time elapsing during the RT. Our aim was to reprocess published data: (1) to compare the shape and amplitude of EMG-locked and stimulus-locked ERPs on correct trials, and (2) to compare the size of the EMG-locked Ne-like waves obtained on fast and slow trials. The results neither support the artifact hypothesis nor the RT monitoring one. Therefore, it seems that the Ne-like waves observed on correct trials do correspond to a Ne, which suggests that the Ne has a broader signicance than just error detection. 2003 Elsevier B.V. All rights reserved.
Keywords: Errors; Ne; ERN; Laplacian; Correct responses
1. Introduction When subjects produce an error in a reaction time (RT) task, a large negative EEG wave called error negativity (Ne; Falkenstein et al., 1991) or error-related negativity
Corresponding author. Present address: Institut de M decine Navale du Service de Sant des Arm es, BP e e e 610, 83800, Toulon Naval, France. Tel.: +33-494099618; fax: +33-494099251. E-mail address: f.vidal@imnssa.net (F. Vidal).
0301-0511/$ see front matter 2003 Elsevier B.V. All rights reserved. doi:10.1016/S0301-0511(03)00097-8
266
F. Vidal et al. / Biological Psychology 64 (2003) 265282
(ERN; Gehring et al., 1993) appears at the midline with a frontocentral maximum. The Ne peaks about 100 ms after the onset of the electromyogram (EMG) of the incorrect response agonist. Several experimental arguments (see Falkenstein et al., 2000 and Coles et al., 2001 for a review) led to the idea that the Ne reects an error-detection process. One of the most important pieces of evidence in favor of this error-detection hypothesis was the specicity of the Ne to erroneous responses. Recently, however, several research groups observed Ne-like waves on correct responses, after computing either an averaged reference (Falkenstein et al., 2000; Luu et al., 2000) or an estimation of the surface Laplacian (Vidal et al., 2000). This Ne-like wave, however, was always smaller than the Ne evoked by errors. A question clearly pointed out by Coles et al. (2001, p. 175) is, if the ERN/Ne is indeed an error-related ERP component, related to error detection, why is it observed on correct trials?. This issue is important because if the Ne is not specic to errors its functional signicance must be reconsidered. Coles et al. (2001), assuming that the Ne does represent an error-detection mechanism, discussed possible reasons for the presence of Ne-like waves on correct trials, as follows. In certain experimental situations, false error detections may occur on correct trials.1 However, these authors admit that this explanation cannot apply to data where subjects had to perform a Go/Nogo task with very rare (10%) Nogo trials (Vidal et al., 2000; Experiment II): The likelihood of committing an error was very low, the stimulusresponse mapping was very simple and compatible, and the stimuli were large and unambiguous as to the behavior they required. To account for the Ne-like wave observed on correct (Go) trials Coles et al. (2001) proposed two possible explanations: 1. There is no true Ne on correct trials. Stimulus-evoked potentials overlap with response-evoked ones, which results in an illusory response-locked negativity. Conditions eliciting short RTs are, of course, especially prone to this kind of artifactual effect. Therefore, in Experiment II of Vidal et al. (2000), the response-locked negative wave observed on correct (Go) responses could result from a contamination by a stimulus-locked negativity. 2. There is a true Ne on correct trials because subjects monitor more parameters of their responses than those explicitly specied by instructions. This causes a discrepancy between an internal (subject-dependent) implicit denition of correct responses and the external (experimenter-dependent) explicit denition of correct responses. An especially relevant additional parameter could be the speed of the response process. Indeed, Luu et al. (2000) have demonstrated that when subjects have to give their response before an RT deadline, the Ne on correct trials increases with RT and is particularly obvious for RTs exceeding the deadline. The same effect had been reported, previously, by Johnson et al. (1997). Therefore, Coles et al. (2001) proposed that the temporal parameter was implicitly monitored by the subjects, i.e. the subjects would have implicitly set an RT deadline beyond which the response was too late. As a consequence, too late responses evoked an error signal resulting in a Ne, whereas soon enough responses did not. Therefore, Vidal et al.s (2000) data are a mixture of objectively correct but subjectively incorrect
1 For instance, when the stimulus is either ambiguous or degraded, or when stimulus-response mapping is incongruent.
F. Vidal et al. / Biological Psychology 64 (2003) 265282
267
(too late) responses, and objectively and subjectively correct (soon enough) ones. The presence of the Ne-like wave in the average of correct trials could be due exclusively to a subgroup of late response trials. The aim of the present work is to evaluate the two hypothesis proposed by Coles et al. (2001) to account for the presence of Ne-like waves on correct responses: averaging artifact and/or monitoring of the temporal parameter (RT) of the response. To achieve this goal, the data obtained in the Go/Nogo task of Experiment II of Vidal et al. (2000) were re-processed as follows: 1. Correct responses were averaged time-locked to the stimulus. If the EMG-locked Ne-like wave obtained on correct responses results from a contamination by stimulus evoked components, these should show up better on stimulus-locked averages than on EMG-locked ones. Moreover, for a negative stimulus-evoked component(s) to be a candidate for such an artifact, its latency should be compatible with the mean premotor time (PMT; PMT is the delay separating stimulus onset and EMG onset). 2. Correct responses were sorted according to their PMT. Correct trials associated with short PMTs were averaged separately from correct trials associated with long PMTs. If, as suggested by Coles et al. (2001), subjects have set an implicit RT deadline, then correct responses associated with short RTs, should elicit smaller Nes than correct responses associated with long RTs, as in the experiments of Luu et al. (2000) and Johnson et al. (1997). 3. The trials were sorted as a function of increasing PMT. Then, raster-like plots of stimuluslocked Laplacians amplitude were constructed, representing the amplitude of the Laplacian as a function of time and as a function of increasing PMTs. Two methods were used: (1) the one published by Jung et al. (2001), and (2) the one used by Coles et al. (2001). This permits a dissociation of stimulus-locked from EMG-locked components.
2. Method 2.1. Subjects There were 12 subjects (11 men, 2 women, from 24 to 50 years old). Written informed consent was given before the experiment. They were all right handed as assessed by the Edinburgh inventory (Oldeld, 1971) and they had a normal or corrected to normal vision. One subject had to be replaced by another one because his data contained too many artefacts. 2.2. Stimuli Stimuli consisted of the words: droite, gauche, toutes and piges (right, left, all and trap in French, respectively). Each word written in white was presented in the center of a faradized video monitor (Stim System of Neuroscan; total of visual angle 1.5 ).
268
F. Vidal et al. / Biological Psychology 64 (2003) 265282
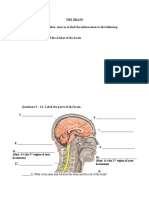
2.3. Task Responses consisted of button presses on a response pad (Stim System of Neuroscan), and had to be made as quickly as possible after the presentation of the words on the screen. 2.4. Trial events There were three pairs of blocks with 80 trials per block: in one pair, the response consisted of a right thumb key-press in response to the word droite, in another pair, the response consisted of a left thumb key-press in response to the word gauche, and in a third pair the response consisted of joint, two thumb key-press in response to the word toutes. The order of pairs of blocks was counterbalanced across subjects. Each block contained 10% catch trials in which the word piges was presented and the subjects had to give no response. In this case, the next RS was given 1300 ms after the presentation of the catch trial. However, there was no uncertainty on the response to be given if required and the uncertainty on the requirement (response or no response) was still low: 10%. Note that, in a counterbalanced order, subjects also performed a three-choice RT task where stimuli, responses and stimulus-to-response mapping where the same as in the task analyzed here, except that there were no catch trials (Vidal et al., 2000 for details). 2.5. Electrophysiological recordings EEG was recorded continuously during the experiment from 10 scalp electrodes (see Fig. 1). The reference and ground were on the right and left mastoids, respectively. Impedances were kept below 5 k .
Fig. 1. Position of the electrodes on the scalp. A nodal electrode (black) and its three surrounding neighbours form a module. FCz is comprised in a module (interelectrode mean distance, 3.7 cm). The reference and the ground are respectively on the right and left mastoid apophysis.
F. Vidal et al. / Biological Psychology 64 (2003) 265282
269
2.5.1. Electrodes placement In order to be able to estimate the time course of surface Laplacian by the source derivation method (Hjorth, 1975), as modied by MacKay (1983), we used an electrode conguration that partly differs from the standard 1020 electrode placement system. This conguration permitted the Laplacian to be estimated at three electrodes-called nodal electrodes. A nodal electrode was surrounded by three other electrodes that formed the vertices of an equilateral triangle, so that the nodal electrode was at the center of that triangle. The distance D between a nodal electrode and its three surrounding electrodes was 1/20th of the inionnasion plus tragustragus distance (i.e. 3.7 cm in average). A nodal electrode and its three surrounding neighbours formed a module. To ensure that the distances and angles between electrodes were kept constant, we used a rigid, three branched, wire template, in which each branch was separated from its neighbouring branch by an angle of 120 . Three electrode modules were constructed which enabled us to place two nodal electrodes on C3 and C4 (about 1 cm medial from C3 and C4) and one nodal electrode on FCz. EEG and EOG signals were fed in Nicolet ampliers, amplied (30 000 times) ltered and digitized (bandwidth: .1100 Hz, 12 dB per octave, sampling rate: 256 Hz). EOG was recorded bipolarly between electrodes situated above the right eye and at its outer canthus. No selective notch 50 Hz lter or additional digital ltering was used. EMG was recorded from the exor pollicis brevis of each hand, by paired surface Ag/AgCl electrodes (6 mm diameter), amplied (5000 times), ltered (high frequency cut-off: 1 kHz), full wave rectied and integrated (integration window: 5 ms), and then digitized (sampling rate: 256 Hz). 2.6. Artifacts rejection Although the use of surface Laplacian has been shown to remove ocular contamination (Law et al., 1993), large ocular artifacts (> 50 V) were rejected on the basis of visual inspection of the monopolar recordings, i.e. the characteristic shape of these artifacts, EOG recordings, and the gradients of activity obtained at different locations. We also carefully rejected local artifacts (i.e. artifacts present at single electrodes: phasic artifacts as well as slow electrical shifts) because the use of the Laplacian transformation enhances them. The remaining monopolar recordings were averaged and Laplacians were calculated on the basis of these monopolar averages. 2.7. Averaging Two kinds of averaging were performed on correct responses. Stimulus-related activities were averaged time-locked to the stimulus and EMG-related activities were averaged time-locked to EMG onset. The onset of EMG activities was detected by visual inspection of each trial (Van Boxtel et al., 1993; Hasbroucq et al., 1999) for further averaging. 2.8. Data processing 2.8.1. Laplacians Under the assumption that the scalp is isotropic, one can demonstrate that the surface Laplacian ( V(x,y) = 2 V(x,y)/x2 + 2 V(x,y)/y2 , where V is the Laplacian of the
270
F. Vidal et al. / Biological Psychology 64 (2003) 265282
electric potential, x and y the cartesian coordinates and V the function of these two variables that describes the spatial distribution of the potential) is proportional to the radial component of the gradient of the current density (also called more shortly scalp current density (SCD) or current source density (CSD)). Intuitively, the Laplacian can be viewed as the local degree of curvature of the scalp potential eld at a given instant. It presents certain advantages: it is reference free, and, acting as a high-pass spatial lter, it removes the blurring effect of the diffusion of the currents through the highly resistive skull (Katznelson, 1981). It is often considered as giving a good approximation of what would be the corticogram (Gevins, 1989). The deblurring effect of the surface Laplacian makes that it greatly reduces the contribution of remote sources to the local recordings. Conversely, it presents some disadvantages: its sensitivity to a source decreases sharply with its depth (Pernier et al., 1988; Manahilov et al., 1992). The surface Laplacian is more sensitive to spatial and electrical noise than monopolar recordings and, in the case of the source derivation method, it needs a regular symmetrical arrangement of the electrodes in order to be correctly approximated. We used the source derivation method (Hjorth, 1975). This method is aimed at calculating an approximation of the surface Laplacian at certain electrodes that we have called nodal. It can be demonstrated that if one approximates the gradient of potential (along one spatial dimension) by the potential differences between two electrodes divided by their distance, then, if a nodal electrode is at the center of a triangle or of a square on which apexes are placed surrounding electrodes, then the Laplacian is proportional to three times the potential at the nodal electrode (four times in the case of a square), minus the sum of potentials at the surrounding electrodes, divided by the square of the inter-electrodes distance. 2.8.2. Fast and slow trails EMG-locked averages corresponding to fast and slow responses were obtained as follows. For each subject, the median PMT value was calculated. Trials were sorted into two groups according to their PMT: shorter or longer than the median PMT value. In order to increase contrast, trials yielding PMTs equal to the median value were dropped. 2.8.3. Raster-like plots 1. A raster-like plot of stimulus-locked Laplacian was constructed as follows (Jung et al., 2001), using the EEGLAB and ICA toolbox software tools: (Delorme et al.: www.cnl.salk. edu/arno/eeglab.html; Makeig et al.: www.cnl.salk.edu/scott/ica.html). Estimation of surface Laplacians was calculated for each accepted trial on the basis of monopolar data. Then, the trial-by-trial Laplacian data were digitally high-pass ltered above 3 Hz in order to eliminate the slow components, which were of no interest for the present study. After ltering, trials containing activities larger than 2.5 V/cm2 were rejected. All the trials of all the subjects were sorted and classed from the shortest to the longest PMTs. Then, bins of 15 adjacent trials were averaged together. All the bins were represented on the same graph in a raster-like plot of stimulus-locked Laplacian changes. On this three-dimensional graph, the Laplacian amplitude, represented by a color scale, is plotted as a function of time, in abscissa, and as a function of increasing PMT, in ordinate.
F. Vidal et al. / Biological Psychology 64 (2003) 265282
271
No error was possible in this task. Therefore, to allow for a comparison between the rasters corresponding to correct responses and those corresponding to errors, we processed in the same way the data corresponding to error trials obtained from the same subjects in a three-choice RT task performed before or after the simple RT task analyzed here, in a counterbalanced order. 2. For the sake of comparison between our data and those of Coles et al. (2001) a second raster-like plot of stimulus-locked data was also constructed by applying exactly the same procedure as that described by Coles et al. (2001) (p.183) except that: (1) the minimal number of trials averaged per 50 ms bin for each subject was 21 or more per subjects instead of 30 or more, and (2) our bins were constructed on the basis of PMTs instead of RTs. We constructed three plots for the correct responses (errors were to few to apply this procedure). The rst plot corresponds to our monopolar data obtained on correct responses. The second plot corresponds to the same data after they have been digitally 3 Hz high-pass ltered (see Luu and Tucker, 2001). The third plot corresponds to the same data after Laplacian transformation.
3. Results 3.1. Behavioral data Mean RT was 329 ms, mean PMT was 251 ms and median PMT was 242 ms. The number of trials yielded by each subjects, after artifact rejection performed on the basis of monopolar recordings, in seven PMT bins is presented in Table 1 . In the same way, we also present, in Table 2, the total number of trials, the median PMT, the mean and
Table 1 Number of trials obtained from each subject (rows 213) and all the subjects (last row), after artefact rejection based on monopolar recordings, in seven PMT bins from the fastest (<150 ms) to the slowest ones (> 400 ms) <150 ms S.B. 24 B.G. 24 S.D. 25 F.C. 25 P.F. 26 F.B. 26 P.L. 27 C.L. 33 Y.D. 36 J.R. 45 J.G. 48 P.G. 50 All subjects 17 22 12 22 28 38 36 25 18 15 9 34 276 150200 ms 39 92 42 41 75 88 112 108 49 64 78 40 828 200250 ms 77 185 63 127 177 126 125 145 135 249 119 64 1592 250300 ms 132 84 103 138 112 75 73 82 139 70 138 106 1252 300350 ms 103 17 105 61 22 59 37 24 39 12 53 76 608 350400 ms 42 5 52 30 7 23 13 6 14 4 13 46 255 > 400 ms 9 5 43 10 9 11 9 6 11 4 7 47 171
The rst column indicates the initials and the age of the subjects from the youngest (24) to the oldest (50).
272
F. Vidal et al. / Biological Psychology 64 (2003) 265282
Table 2 This table presents for each subject (rows 213) and all the subjects (last row) the total number of trials obtained after artefact rejection based on monopolar recordings (second column), the median PMT (third column), the mean (fourth column) and the standard deviation (fth column) of the PMTs, and the percentage of trials occurring in a 200300 ms premotor bin (last column) Number of trials S.B. 24 B.G. 24 S.D. 25 F.C. 25 P.F. 26 F.B. 26 P.L. 27 C.L. 33 Y.D. 36 J.R. 45 J.G. 48 P.G. 50 All subjects 419 410 420 429 430 420 405 396 405 416 417 413 4982 Median PMT 277 230 293 254 227 234 223 223 250 227 250 285 242 Mean PMT 274 229 293 260 234 243 230 228 252 227 250 286 250.5 Standard deviation 66 51 78 64 60 79 70 61 66 40 57 97 65.75 200300 (%) 50 66 40 62 67 48 49 57 68 76 62 41 57
the standard deviation of the PMTs, and the percentage of trials occurring in a 200300 ms PMT bin. From Table 1, it appears that, (1) except for the slowest subject (S.D.), the largest number of responses corresponds to PMTs included in the 200250 ms or in the 250300 ms bin, and (2) these two bins do not correspond to a fast or a slow tail of the distribution in any subject. From Table 2 it appears that, depending on the subjects, the 200300 ms PMT bin contains from 40% of the trials (slowest subject S.D.) to 76% of the trials (fastest subject J.R.). Moreover, all the means and medians of the subjects are comprised in this 200300 ms PMT bin. Therefore, this 200300 ms bin does not contain the slowest or the fastest tail of the distribution for any subject but, rather, corresponds to median values of PMTs in all the subjects. 3.2. Electrophysiological data The surface Laplacian data presented here were obtained by the source derivation method (Hjorth, 1975) modied by MacKay (1983). Fig. 2 represents stimulus-locked (a) and EMG-locked (b) grand averages for correct responses (same scale). In this gure, the size of the stimulus-locked N1 is smaller than the size of the EMG-locked Ne; moreover, the stimulus-locked N1 occurs too early to account for the presence of any EMG-locked negativity (see also Figs. 3a and 4c). As the observed mean differences already negate the contamination view, no statistical analyses are required. Fig. 3a represents raster-like plots of stimulus-locked Laplacian changes (color scale) as a function of time (abscissa) and as a function of increasing PMTs (ordinate) for the correct responses of the task analyzed here (simple RT task in Experiment II of Vidal et al., 2000). The procedure used here is that described by Jung et al. (2001). The vertical black line indicates the moment of stimulus onset, the S-shaped black line represents the time of
F. Vidal et al. / Biological Psychology 64 (2003) 265282
273
Fig. 2. Amplitude of surface Laplacian estimated at FCz (ordinate, in V/cm2 ) as a function of time (abscissa, in ms) obtained on correct responses. (a) time-locked to stimulus onset (0 of time: vertical line); (b) time-locked to EMG onset (0 of time: vertical line).
EMG onsets. This gure conrms the interpretations drawn on the basis of Figs. 2a and b: a negative wave, time-locked to the stimulus, occurs in the time window of the N1. Another wave, clearly time-locked to EMG onset follows the usual S-shape distribution of PMTs. In Fig. 3b one can compare the same raster-like plot for errors committed by the same subjects in a three-choice RT task (choice RT task in Experiment II of Vidal et al., 2000). Although there are much less trials in the case of errors, a Ne with a large amplitude appears time-locked to EMG onset. EMG onset latency is shorter for correct responses because they are emitted in a simple RT situation, which was not the case for the errors. Fig. 4 represents raster-like plots of stimulus-locked EEG changes (color scale) as a function of time (abscissa) and as a function of increasing PMTs (ordinate) for the correct responses of the task analyzed here (simple RT task in Experiment II of Vidal et al., 2000). The procedure used is the one described by Coles et al. (2001). Fig. 4a corresponds to our monopolar data; Fig. 4b represents the same monopolar data, after they have been digitally 3 Hz high-pass ltered; Fig. 4c corresponds to the same data, after they have been Laplacian
274
F. Vidal et al. / Biological Psychology 64 (2003) 265282
Fig. 3. Raster-like plot of stimulus-locked Laplacian changes (procedure of Jung et al., 2001). On this three-dimensional graph, the Laplacian amplitude, represented by a color scale (scale: tenth of V/cm2 ), is plotted as a function of time (in abscissa: ms), and as a function of increasing PMTs (in ordinate); (a): correct responses in the simple RT task; (b): errors committed by the same subjects in a similar three-choice RT task. The vertical black lines indicate the moment of stimulus onset, the S-shaped black lines indicate the time of EMG onsets.
F. Vidal et al. / Biological Psychology 64 (2003) 265282
275
Fig. 4. Raster-like plots of stimulus-locked EEG changes (procedure of Coles et al., 2001) on correct responses. On this three-dimensional graph, EEG amplitude, represented by a color scale (a and b: V; c: tenth of V/cm2 ), is plotted as a function of time (in abscissa: ms), and as a function of PMTs (in ordinate: ms). (a) original monopolar data; (b) monopolar high-passed ltered (3 Hz) data; (c) Laplacian transformed data. The vertical white lines indicate the moment of stimulus onset, the tilted dashed white lines indicate the time of EMG onsets.
transformed. The vertical white lines indicates the moment of stimulus onset, the tilted dashed white lines indicate the time of EMG onsets.2 In Fig. 4a no clear negative wave time-locked to EMG onset can be evidenced. In Fig. 4b, the 3 Hz high-pass ltering has partially unmasked an EMG-locked negative-going wave. Fig. 4c conrms the interpretations drawn on the basis of Figs. 2a and b and 3a: a negative wave, time-locked to the stimulus, occurs in the time window of the N1. Another negative wave, clearly time-locked to EMG onset follows the linear evolution of PMTs.
2 The lack of continuity between the elements of the tilted line result from technical limitation and do not reveal discontinuity in the data.
276
F. Vidal et al. / Biological Psychology 64 (2003) 265282
Fig. 5. Amplitude of surface Laplacian estimated at FCz (ordinate, in V/cm2 ,) as a function of time (abscissa, in ms) obtained on correct responses, time-locked to EMG onset (0 of time: vertical line). Thin lines correspond to PMTs shorter than the median value of PMTs, bold lines correspond to PMTs longer than the median value of RTs.
Fig. 5 represents EMG-locked averages corresponding to correct responses. Fast and slow trials are averaged separately. This gure shows that, in this experiment, the amplitude of the Ne is not related to the PMT duration of the corresponding response.
4. Discussion 4.1. Reciprocal contamination of stimulus-locked and EMG-locked averages The comparison between stimulus-locked and EMG-locked averages (Figs. 2a and b) indicates that the Ne-like wave occurring on correct trials cannot be accounted for by an averaging artifact, that is a contamination of EMG-locked averages by stimulus-locked ERPs. Figs. 3a and 4c also indicate that the Ne-like wave obtained on correct responses does not result from an overlap between stimulus-locked and EMG-locked EEG activities. On the contrary, Figs. 3a and 4c suggest that, when comparing stimulus-locked data leading to different RTs, one must be cautious in interpreting stimulus-locked amplitude differences. For instance, many responses occur in the time window of the P300; therefore, EMG-locked negativities might partly cancel the P300, reducing its apparent amplitude. When comparing the amplitude of the P300 between conditions leading to different RTs (or conditions with and without a response), certain P300 differences might be simply due to partial cancellation by EMG-locked negativities. This is in line with the results of Salisbury et al. (2001). These authors studied the amplitude and topography of the P300 in button pressing and silent counting. They suggested that the reduced amplitude of the P300 in button pressing was due to a contamination by EMG-locked components. 4.2. Implicit monitoring of the temporal parameter More important is the fact that the amplitude of the Ne obtained on correct trials is not larger as PMT get longer (Figs. 3a, 4c and 5). Luu et al. (2000) and Johnson et al. (1997)
F. Vidal et al. / Biological Psychology 64 (2003) 265282
277
obtained a Ne on correct trials which showed maximal amplitude when the RTs exceeded a deadline. The present analysis indicates that, contrary to Coles et al.s (2001) hypothesis, the modulation of the size of the Ne as a function of RT on correct trials (Johnson, 1997; Luu et al., 2000) is the result of explicit (not implicit) setting of such a deadline. The presence of a Ne on correct trials in the present experiment cannot be accounted for by a mismatch between subjects evaluation of the ongoing RT and an implicit deadline. Pailing et al. (2000, p. S76) did report Ne-like waves on correct responses for long RTs in a task where no explicit RT deadline was given. However, after they analyzed the correlation between the amplitude of this Ne-like wave and the size of the preceding P300, these authors concluded that . . . the greater ERN-like component for slow responses may be an artifact of large RT variability for slow responses and the consequent P300 amplitude reduction.3 To reconcile the present data with Coles et al. (2001) opinion, one should add a supplementary hypothesis: the implicit deadline varies from trial to trial (remember that the subjects of Luu et al. received a feedback on each trial). However, this explanation would be ad hoc and, to our knowledge, there is no available data supporting (or excluding) this possibility. Another possibility would be that, in absence of explicit requirement, responses that are too fast might also be considered as errors, for example, if subjects make fast guesses. If one considers the 200300 ms PMT bin of Fig. 3a, it is clear from Tables 1 and 2 that this PMT bin does not correspond to the fastest or the slowest tails of the distribution of PMTs in any subject but, rather, to median PMT values. In this bin, however (extending roughly from trials 10003800), a negative wave appears in Fig. 3a. In Fig. 4c, all the subjects contribute equally to each bin of the distribution. In this gure, there is no evidence that the negative wave observed on correct responses disappears for the middle latencies of the distribution. Therefore, even in middle latency responses does a negative wave occur, time-locked to EMG onset. 4.3. Ne on correct responses: a mandatory component? Now, although Coles et al. (2001) did not discuss this possibility, a possible way to reconcile the existence of a Ne on correct trials with the error-detection hypothesis would be to consider that the Ne-like wave observed on correct trials is not a true Ne but corresponds to another EMG-locked mandatory component, independent from the Ne. This component would be present on all trials (correct or incorrect). On errors, another true Ne would add to this mandatory component. The EMG-locked negativity observed on errors would result from the summation of two waves: a mandatory EMG-related component plus an error-related one. This possibility, however, does not seem to be supported by data. Vidal et al. (2000), on the basis of CSDs maps showed comparable distributions for the negative wave elicited on correct trials, the Ne evoked on errors and the Ne evoked after incorrect EMGs. With more sophisticated methods, Luu and Tucker (2001) compared in great details the temporal and spatial properties of the Ne obtained on correct responses and on errors. These authors, observed that most of the RTs produced by the subjects occurred in the time window of the P300. This led them to consider that very large activities like
3 Note that such an artefact cannot account for the data reported by Luu et al. (2000) because these authors ltered their data, in the time domain, in order to eliminate the P300 component.
278
F. Vidal et al. / Biological Psychology 64 (2003) 265282
those evoked by erroneous trials showed up very clearly because of their size. However, they suggested that smaller EMG-locked activities such as the Ne corresponding to correct responses could be dampened by the superimposition of a stimulus-locked P300 (this argument is symmetrical to one made by Coles et al.). The time constant of the P300 being larger than that of the Ne, these authors ltered their data (412 Hz band pass) for both correct and erroneous responses. After ltering, the waveforms corresponding to Ne obtained on correct and incorrect responses (interpreted by the authors as a part of midline frontal rhythm) were compared. The Ne was especially prominent on error trials but was also present, although smaller, on correct responses. However, the Nes corresponding to correct responses and to errors were not different regarding their latency, their scalp distribution (averaged reference: 128 electrodes), the position of their estimated sources (with brain electrical source analysis; BESA), and their source solutions derived from the weighted minimum norm method. In spite of the large number of electrodes used to map the Ne, and of the different independent signal processing methods used by Luu and Tucker (2001) to analyze the Ne on correct and incorrect responses, it was not possible to nd any evidence that the Ne elicited by correct responses and by errors were different components. Therefore, the most parsimonious position to account for the available data seems to be that: (1) There is an EMG-locked Ne-like wave on correct responses, and (2) This Ne-like wave can be distinguished from the Ne evoked by erroneous responses neither on its temporal nor on its spatial properties, which suggest that the Ne-like and the true Ne are of same nature. Moreover, the data presented here indicate that this Ne is not due to an implicit monitoring of the temporal (RT) parameter. This makes problematic the interpretation of the Ne in terms of error detection. Luu et al. suggested that the Ne is part of an oscillatory potential such as a midline frontal theta rhythm. The present data support this view. In Fig. 2, the Ne and the negative wave which follows it (peak around: 300 ms) can be viewed as the negative side of an oscillation in the band. The same interpretation can be drawn from Fig. 3a, after EMG onset. 4.4. Observations on patients Observations on patients do not t very well with the error-detection hypothesis, either. Gehring and Knight (2000) recorded EEG activity of patients with unilateral prefrontal lesions (PFC) performing an RT task. In these patients, the Ne evoked by correct responses was as large as that evoked by errors. The same observations hold for schizophrenic patients (Ford, 1999), whose symptoms are often related to abnormal prefrontal functions. Coles et al. (2001) argued that this effect could be explained if one assumes that, in these patients, the representation of the appropriate response is unavailable to the comparator. In such a case, a Ne would always occur whether the response is correct or not.4 This suggests that the nervous system has no access to the status of the ongoing response. This viewpoint, however, is challenged by two kinds of observations.
4 Note that if frontal dysfunction unmasks a large Ne on correct responses instead of diminishing the Ne on errors, then the large Ne on errors corresponds to the process by default. The small Ne observed on correct responses would result from an inhibition of the structure generating the Ne by the prefrontal cortex.
F. Vidal et al. / Biological Psychology 64 (2003) 265282
279
1. While Ford (1999) did not report the performance of her patients, Gehring and Knight (2000) did. The reduction of response force on error trials (as compared to correct responses) was smaller in patients than in aged matched and young subjects. Now, considering the complexity of the task, it is remarkable that the error rate did not differ between the patients and the two control groups (aged matched and young subjects). The post-error slowing (longer RTs on correct responses following an error: Rabbitt, 1966) did not differ either between patients and aged matched control subjects. Moreover, as pointed out by Gehring and Knight (2000, p. 518) although the PFC group showed an unusually large ERN on correct trials, they did not correct their correct responses more often than did the controls. How is it possible that patients slow down their RT after an error as well as normal subjects do, and do not correct their correct responses more often than the controls do, if no representation of the correct response is available? 2. Although Ford (1999) did not discuss this point, her data show a large error positivity (Pe; Falkenstein et al. 1991) on errors in control subjects and in schizophrenic patients (Fig. 10, p. 679). The Pe is completely absent on correct responses in control subjects and in schizophrenic patients. Although the traces shown by Gehring and Knight (2000) stopped shortly after the resolution of the Ne, a close inspection of the data corresponding to their patients (Fig. 2, p. 518) shows that, as soon as 100 ms, after the erroneous response, a steep positive wave begins to develop whereas the potentials are at the baseline level on correct responses. Therefore, the patients of Ford (1999) and probably those of Gehring and Knight (2001) present a Pe on errors only. How is it possible that a Pe is elicited, on errors only, if no representation of the correct response is available? Now, if a correct representation of the correct response is actually available, and if the Ne represents the output of a comparison process, how is it possible that the Ne is as large on correct responses as it is on errors? In light of the present analysis and of recent available data from the literature, it seems difcult to maintain that the Ne reveals error detection, that is the outcome of a comparison process, rather than the activity of this comparison process itself. 4.5. A conict monitoring account of the Ne? Recently, a new account of the Ne has been proposed by Botvinick et al. (2001), the conict monitoring model. Basically, their model is a three-layered connectionist model, one layer for stimulus representations, one layer for response representations, and the last one, the attentional module, represents the role of attention on perceptual processes. To these three layers model, Botvinick et al. (2001) added a conict monitoring module which continuously evaluate the degree of conict between responses. The conict is the product of the activations of the two responses, scaled by the strength of the inhibitory connection between them. By mean of simulations, the authors showed that the degree of conict in various tasks and experimental conditions correlates with the Ne amplitude recorded in these situations. The presence of a Ne-like on correct trials in simple RT is a challenge for this version of the conict model, even if we consider that the very few Nogo trials could induce a conict
280
F. Vidal et al. / Biological Psychology 64 (2003) 265282
between respond and not respond (Nieuwenhuis et al., 2003). Indeed, Nieuwenhuis et al. (2003) recently applied the conict hypothesis to account for the EEG activities in the GoNogo task. Their idea was that not respond can be considered as a response, and hence that a conict can occur between the two response representations. However, they interpreted the N200 as an index of the conict in correct responses, that is an activity occurring before the response (see also Botvinick et al., 2001, p.635), whereas the Ne in the present study clearly occurs after the response. Therefore, if we follow Nieuwenhuis et al.s conclusions, the conict in the GoNogo task occurs before the response, and hence the Ne-like wave cannot be interpreted as a conict. One may argue, in line with Cohen et al. (2001), that a conict occurs after the correct response because the subjects had a poor representation of the two responses (Go and Nogo), inducing a post-response activation of the Nogo representation even after the response in the Go trials. Although we cannot exclude such a possibility, it seems very unlikely as we see no reasons why it did not occur also in Nieuwenhuis et al.s data. Therefore, the presence of a Ne-like wave on correct trials in the present task is not easily explained by the conict model. Finally, one cannot exclude a possibility put forward by several authors (Tucker et al., 1999; Gehring and Knight, 2000; Luu et al., 2000; Vidal et al., 2000; Gehring and Fencsik, 2001) that the Ne represents an emotional or affective reaction. 4.6. Methodological aspects The fact that the Ne on correct responses often goes unnoticed raises questions about its reliability. One explanation may be found in Luu and Tucker (2001). As mentioned earlier, these authors, noticed that most of their responses occurred in the time window of the P300. They supposed that the small Nes (on correct trials) might be masked by a superimposition of the P300. On errors, the large size of the Ne would be sufcient to show up despite the P300 masking effect. Therefore, they high-pass ltered their data in the temporal domain, to remove, at least in part, the P300 effect. Indeed, the Ne showed up more clearly after such a processing. Thus, removing a part of the raw data unmasked or at least enhanced the Ne on correct trials. In a certain sense, the Laplacian transformation produces the same effect: it acts as a high-pass lter in the spatial domain. As a spatial lter, the Laplacian transformation does not create new data but removes part of them. By removing some components it unmasks the remainder. In the same line of reasoning as that of Luu and Tucker (2001), one can suppose that, if the P300 and/or the resolution of the CNV, cancel the Ne on correct trials and if they are not generated beneath FCz, high-pass spatial ltering removes or strongly attenuates them. These two ltering methods (temporal and spatial) are aimed at the same goal, unmasking small components overlapped by larger ones. This is achieved by removing larger components generated in another time domain (temporal ltering) or by removing other components generated in another spatial domain or area (spatial ltering). While the Laplacian transformation is one of the available spatial ltering methods, it is not the only one. For instance, the averaged reference is another method but it is less efcient (Mac Farland et al., 1997). Nonetheless, in the case of the Ne on correct trials, this method has proved to be useful (see Falkenstein et al., 2000).
F. Vidal et al. / Biological Psychology 64 (2003) 265282
281
The comparison of Figs. 4a, 4b and 4c is consistent with our interpretation. Fig. 4a shows a negative wave time-locked to the stimulus occurring in the time window of the N1. After the response, a large slow positive wave develops for at least 300 ms. A small decrease in this positive activity can be suspected especially for the shortest and the slowest RTs. A close examination of Coles et al. data shows the same kind of effects, especially for the shortest and the slowest RTs, although the data are collected in a very different paradigm. Therefore, our monopolar data and Coles et al.s ones show very similar patterns. Now, after 3 Hz high-pass ltering (temporal ltering) the same monopolar data, a procedure comparable to that used by Luu and Tucker (2001), we did unmask, at least in part, a negative component time-locked to EMG onset (Fig. 4b). Finally, after Laplacian transformation of the monopolar data (spatial ltering), we unmasked a negative wave clearly time-locked to EMG onset (Fig. 4c). These results justify our processing and that of Luu and Tucker (2001) in order to observe properly the negative wave elicited after correct responses. In conclusion, the presence of Ne-like waves on correct responses results neither from a contamination of EMG-locked activities by stimulus-locked ones, nor from an implicit monitoring of the time elapsing during the RT. Therefore, the Ne-like and the true Ne are likely of same nature. As a consequence, the current models accounting for the generation of the Ne should take into account this empirical fact.
Acknowledgements We are very grateful to Dominique Reybaud for her technical contribution and to Sonia Allain for her help in data processing. This research was supported by grant cognitique ACT 54b from the Ministre de la Recherche and by grant 99-CO-02 from the Dlgation Gnrale lArmement. The authors are indebted to W.A. MacKay for language editing and comments.
References
Botvinick, M.M., Braver, T.S., Barch, D.M., Carter, C.S., Cohen, J.D., 2001. Conict monitoring and cognitive control. Psychol. Rev. 108, 624652. Cohen, J.D., Botvinick, M., Carter, C., 2001. Anterior cingulate and prefrontal cortex: whos in control?. Nat. Neurosci. 3, 421423. Coles, M.G.H., Scheffers, M.K., Holroyd, C.B., 2001. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and theory of error-processing. Biol. Psychol. 56, 173189. Falkenstein, M., Hohnsbein, J., Hoormann, J., 1991. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction time tasks. Electroencephalogr. Clin. Neurophysiol. 78, 447455. Falkenstein, M., Hoormann, J., Christ, S., Hohnsbein, J., 2000. ERP components on reaction errors and their functional signicance: a tutorial. Biol. Psychol. 51, 87107. Ford, J.M., 1999. Schizophrenia: the broken P300 and beyond. Psychophysiology 36, 667682. Gehring, W.J., Fencsik, D.E., 2001. Functions of the medial frontal cortex in the processing of conict and errors. J. Neurosci. 21, 94309437. Gehring, W.J., Knight, R.T., 2000. PrefrontalCingulate interactions in action monitoring. Nat. Neurosci. 3, 516520.
282
F. Vidal et al. / Biological Psychology 64 (2003) 265282
Gehring, W.J., Goss, B., Coles, M.G.H., Meyer, D.E., Donchin, E., 1993. A neural system for error detection and compensation. Psychol. Sci. 4, 385390. Gevins, A.S., 1989. Dynamic functional topography of cognitive tasks. Brain Topogr. 2, 3756. Jung, T.-P., Makeig, S., Westereld, M., Townsend, J., Courchesne, E., Sejnowski, T.J., 2001. Analysis and visualization of single-trial event-related potentials. Hum. Brain Mapping 14, 166185. Hasbroucq, T., Possama, C.A., Bonnet, M., Vidal, F., 1999. The effect of irrelevant location of the response signal on choice reaction time: an electromyographic study in man. Psychophysiology 36, 522526. Hjorth, B., 1975. An on-line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr. Clin. Neurophysiol. 39, 526530. Johnson, T.M., Otten, L.J., Boeck, K., Coles, M.G.H., 1997. Am I too late? The neural consequences of missing a deadline. Psychophysiology 34, S48. Katznelson, R.D., 1981. EEG recording, electrode placement, and aspects of generator localization, in: Nuez, P.L. (Ed.), Electric Fields of the Brain. Oxford University Press, New York. Law, S.K., Nuez, P.L., Wijesinghe, R.S., 1993. High-resolution EEG using spline generated surface Laplacians on spherical and ellipsoidal surfaces. IEEE Trans. Biomed. Eng. 40, 145153. Luu, P., Tucker, D.M., 2001. Regulating action: alternating activation of midline frontal and motor cortical networks. Clin. Neurophysiol. 112, 12951306. Luu, P., Flaisch, T., Tucker, D.M., 2000. Medial frontal cortex in action monitoring. J. Neurosci. 20, 464469. MacKay, D.M., 1983. On-line source density computation with a minimum of electrodes. Electroencephalogr. Clin. Neurophysiol. 56, 696698. Mac Farland, D.J., McCane, L.M., David, S.V., Wolpaw, J.R., 1997. Spatial lter selection for EEG-based communication. Electroencephalogr. Clin. Neurophysiol. 103, 386394. Manahilov, V., Riemslag, F.C., Spekreijse, H., 1992. The Laplacian analysis of the pattern onset response in man. Electroencephalogr. Clin. Neurophysiol. 82, 220224. Nieuwenhuis, S., Yeung, N., van den Wildenberg, W., Ridderinkhof, K.R., 2003. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conict and trial-type frequency. Cogn. Affect. Behav. Neurosci. 3, 1726. Oldeld, R.C., 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97113. Pailing, P.E., Segalowitz, S.J., Davies, P.L., 2000. Speed of responding and the likelihood of error-like activity in correct trials. Psychophysiology 37(1), S76. Pernier, J., Perrin, F., Bertrand, O., 1988. Scalp current density elds: concepts and properties. Electroencephalogr. Clin. Neurophysiol. 69, 385389. Rabbitt, P.M.A., 1966. Errors and error correction in choice-response tasks. J. Exp. Psychol. 71, 264272. Salisbury, D.F., Rutherford, B., Shenton, M.E., McCarley, R.W., 2001. Button-pressing affects P300 amplitude and scalp topography. Clin. Neurophysiol. 112, 16761684. Tucker, D.M., Hartry-Speiser, A., McDougal, L., Lure, P., deGrandpre, D., 1999. Mood and spatial memory: emotion and the right hemisphere contribution to spatial cognition. Biol. Psychol. 50, 103125. Van Boxtel, G.J.M., Geraats, L.H.D., Van den Berg-Lessen, M.M.C., Brunia, C.H.M., 1993. Detection of EMG onset in ERP research. Psychophysiology 30, 405412. Vidal, F., Hasbroucq, T., Grapperon, J., Bonnet, M., 2000. Is the error negativity specic to errors? Biol. Psychol. 51, 109128.
Вам также может понравиться
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Understanding Cerebral PalsyДокумент22 страницыUnderstanding Cerebral PalsyJay SorianoОценок пока нет
- 12.1 Guided ReadingДокумент2 страницы12.1 Guided ReadingGrant HasletonОценок пока нет
- Ericsson PDF Handbook ExpertiseДокумент1 страницаEricsson PDF Handbook ExpertiseEdgarОценок пока нет
- MatsumotoK PS2016 PostSymposium HandoutДокумент4 страницыMatsumotoK PS2016 PostSymposium Handoutdeemoney3100% (2)
- Play TherapyДокумент23 страницыPlay TherapyPuspa WangiОценок пока нет
- Lesson 1 Nervous SystemДокумент3 страницыLesson 1 Nervous SystemDwayneGeraldОценок пока нет
- A Clinician's Approach To Peripheral NeuropathyДокумент12 страницA Clinician's Approach To Peripheral Neuropathytsyrahmani100% (1)
- BTECH Program SyllabusДокумент41 страницаBTECH Program SyllabusAthira SurendranОценок пока нет
- Planning Classes on First Aid for BurnsДокумент2 страницыPlanning Classes on First Aid for BurnsSamielle Alexis GarciaОценок пока нет
- Employee Morale and MotivationДокумент10 страницEmployee Morale and MotivationDianne Maru FlorОценок пока нет
- Substance Abuse BrochureДокумент2 страницыSubstance Abuse Brochureapi-285178079Оценок пока нет
- Neural DarwinismДокумент19 страницNeural Darwinismava939Оценок пока нет
- Is There A Difference Between Hearing and ListeningДокумент3 страницыIs There A Difference Between Hearing and ListeningMuizzuddin RosliОценок пока нет
- Xaccteach PrelimsДокумент13 страницXaccteach PrelimsTintin AquinoОценок пока нет
- Constructing A TPB QuestionnaireДокумент14 страницConstructing A TPB QuestionnaireRodrigo Uribe BravoОценок пока нет
- Nervous System WorksheetДокумент3 страницыNervous System WorksheetaОценок пока нет
- Learner Autonomy in The Internet-Based Language Instruction ContextДокумент3 страницыLearner Autonomy in The Internet-Based Language Instruction ContextChrisОценок пока нет
- Journal Substance AbuseДокумент7 страницJournal Substance AbuseParas AzamОценок пока нет
- AI Session 2Документ64 страницыAI Session 2Gunjan SumanОценок пока нет
- Revising Bloom's Taxonomy to Better Reflect CognitionДокумент7 страницRevising Bloom's Taxonomy to Better Reflect CognitionalmorsОценок пока нет
- Worksheet Psychology Answer The Following QuestionsДокумент8 страницWorksheet Psychology Answer The Following QuestionsAkshayaОценок пока нет
- Influence of Emotional Buying Behaviour On FMCG Products A Case Study On Pathanjali Products in Srikakulam District, AP.Документ8 страницInfluence of Emotional Buying Behaviour On FMCG Products A Case Study On Pathanjali Products in Srikakulam District, AP.archerselevatorsОценок пока нет
- Neurosurgery - Specific Considerations - Schwartz's Principles of Surgery ABSITE and Board Review, 9th EdДокумент17 страницNeurosurgery - Specific Considerations - Schwartz's Principles of Surgery ABSITE and Board Review, 9th Edvnnv101.raceacОценок пока нет
- The Handwriting Book FinalДокумент57 страницThe Handwriting Book Finaljessica-clare.davisОценок пока нет
- General Biophysics Lecture 1: Introduction to Biophysics and Electricity in the BodyДокумент20 страницGeneral Biophysics Lecture 1: Introduction to Biophysics and Electricity in the BodyashrafarafaОценок пока нет
- Tvps-Iii PresentationДокумент48 страницTvps-Iii Presentationapi-290117367100% (3)
- The POQAДокумент1 страницаThe POQADanuSusantoОценок пока нет
- Emotional: IntelligenceДокумент9 страницEmotional: IntelligenceMarkus LandingtonОценок пока нет
- Self Help For PanicДокумент10 страницSelf Help For PanicIqbal Baryar100% (1)
- PPR Koumakis Dementia Care FrameworksДокумент15 страницPPR Koumakis Dementia Care FrameworksLuis Eduardo Ronquillo OrdoñezОценок пока нет