Академический Документы
Профессиональный Документы
Культура Документы
Paper 1
Загружено:
Neural Spark Physics CieИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Paper 1
Загружено:
Neural Spark Physics CieАвторское право:
Доступные форматы
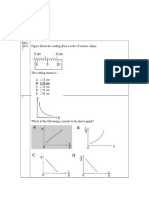
1.
Diagram shows a syringe is connected to a pipe and manometer. The levels of water in each side of the manometer are the same.
Which of the following statements is correct when the water level at P is higher than Q? A. The piston is pressed downward. B. The piston is pulled upward. C. The syringe is moved downward. D. The syringe is moved upward. 2. Diagram shows a mercury barometer
Height of the mercury column, h will increase if A. a bigger diameter capillary tube is used. B. more mercury is inserted in the beaker. C. the barometer is placed at a lower altitude. D. the vacuum in the tube is filled with air. 3. Diagram shows a bottle contains mercury.
The pressure exerted by the mercury at point P is 8.16 104 N m-2 . What is the depth of P if the density of mercury is 1.36 104 kg m-3 ? A. 0.17 m B. 0.60 m C. 1.70 m D. 6.00 m 4. Alcohol is sometimes used as a thermometric liquid because of its A. low density. B. low freezing point. C. ability to wet the glass tube. D. high specific heat capacity. 5. Diagram shows a beaker contains water at room temperature under an inverted bell jar.
When the air is pumped out from the jar, soon the water starts to boil. It is because A. the pressure in the jar decreases and the boiling point of the water increases. B. the pressure in the jar decreases and the boiling point decreases. C. the volume of the air decreases and the boiling point increases. D. the volume of the air decreases and the boiling point decreases. 6. Complete the following statement. The heat absorbed by 10 g of ice to melt completely is equals to A. the heat absorbed by 10 g of steam to condense completely. B. the heat released by 10 g of steam to condense completely. C. the heat absorbed by 10 g of water to evaporate completely. D. the heat released by 10 g of water to solidify completely. 7. What is the final temperature when 100 g of water at 25 oC is mixed with 75 g of water at 50oC ? A. 35.7 oC B. 37.5 oC C. 46.4 oC D. 48.0 oC
Diagram shows a ping pong ball is placed at one end of a spring which is compressed and then released. The ball moves a distance x before it stops.
Which of the following will increase the distance x? A Use longer spring B Use softer spring C Use two identical springs arranged in series D Use spring which has smaller diameter of the spring 9 Diagram shows a constant force, F, acts on a block placed on a smooth surface.
The block will move with A constant speed B constant acceleration C gradually decreasing acceleration D gradually increasing acceleration 10 Diagram shows a block of mass 3 kg supported by a light string on a smooth and frictionless incline plane.
What is the tension in the string if the block is at rest? A 10.0 N B 12.0 N C 15.0 N D 30.0 N 11 Diagram shows three wooden blocks P,Q and R are pressed into the water until its base reaches at level S. The pressure caused by the water at base of the wooden blocks P , Q and R are P1, P2 and P3 respectively.
Which statement is correct? A P1 > P2 > P3 B P1 < P2 < P3 C P1 = P2 = P3 D P1 > P2 > P3 12 An air bubble is at a depth of 5 metre below the surface of a lake. What is the pressure of the bubble due to the depth of water? (Given the density of the water is 1 000 kg m-3 ) A 5 x 10-2 Pa B 5 x 10-3 Pa C 5 x 10 3 Pa D 5 x 104 Pa Gas pressure exists because the gas molecules A move randomly and freely B move at the same velocity C collides with the wall of the container produces change of momentum D collides with one another in elastic collisions and with the walls of the container Diagram shows a cylinder contains air.
13
14
When the piston is pulled upwards, which of the following is true? A The density of the gas molecules increases B The distance between molecules of gas increases C The number of the gas molecules per unit volume increases D The frequency of collisions between the gas molecules with the walls of the container increase 15 Which of the following shows the height h, of simple mercury barometer that measures the atmospheric pressure?
16
In very cold weather, water pipes sometimes burst because A the pipes contract on cooling so that the water pressure inside rises B the ice which formed inside the pipes expands when it melts C water expands when cooled from 4 0C to 0 0C or below. D the formation of ice from water produces an increase in volume.
17
When an ice cube, initially at 0 0C, is left in an area at room temperature, the ice cube will melt because A no energy is required for this process to occur B the molecules within the ice lose kinetic energy C the molecules within the ice gain potential energy D the molecules within the ice lose potential energy
18
Hydroelectric, tidal and fossil fuels are three sources of energy. Which of these are renewable energy sources?
19
Diagram shows a manometer. Liquid B does not mix with water.
Given that the density of water is 1 000 kg m-3 . What is the density of the liquid B? A 480 kg m-3 B 750 kg m-3 C 800 kg m-3 D 1 000 kg m-3 20 Diagram shows water rising up the tubes X, Y and Z if the air is blown.
Which of the following is true regarding the water level in tube X, Y and Z? A Water level in X < Water level in Y < Water level in Z B Water level in X < Water level in Z < Water level in Y C Water level in Z < Water level in Y < Water level in X D Water level in Y < Water level in Z < Water level in X
21
Diagram shows a manometer is connected to a gas supply.
Pressure can be measured in cm of water. What is the pressure of the gas? A 8 cm of water more than atmospheric pressure B 12 cm of water more than atmospheric pressure C 8 cm of water less than atmospheric pressure D 12 cm of water less than atmospheric pressure 22 Diagram shows a polystyrene block being pushed to a depth of h cm from the water surface. P is the water pressure acting on surface Q.
Which graph shows the relationship between P and h when the block is pushed deeper into the water?
23
Diagram shows a student places his thumb firmly on the outlet of a bicycle pump, to stop the air from coming out.
What happens to the pressure and to the volume of the trapped air as the pump handle is pushed in?
24
A 400 W heater is used to heat 0.4 kg of solid. Diagram 40 shows the temperature time graph of the substance.
What is the specific latent heat of fusion of the substance? A 64 kJ kg-1 B 160 kJ kg-1 C 400 kJ kg-1 D 600 kJ kg-1 25 Heat energy is supplied at the same rate to 100 g of paraffin and to 100 g of water in similar containers. Why does the temperature of the paraffin rise more quickly? A The paraffin has a larger specific heat capacity than water B The paraffin has a smaller specific heat capacity than water C The paraffin is less dense than water D The paraffin is more dense than water 26 Bubbles of gas, escaping from the mud at the bottom of a deep lake, rise to the surface.
As the bubbles rise they get larger. Why does this happen? A The atmospheric pressure on the bubbles decreases B The atmospheric pressure on the bubbles increases C The water pressure on the bubbles decreases D The water pressure on the bubbles increases 27 Diagram 1 shows some air is trapped in a cylinder. Diagram 2 shows the same air after the piston has been pushed in slowly.
Diagram 1 The air in diagram 1 is at atmospheric pressure PA. What is the pressure of the trapped air in Diagram 2.
Diagram 2
28
For a fixed mass of gas at constant volume in an air tight container, which of the graphs correctly represents the relationship between pressure and temperature of the gas?
29
Which of the instruments applies the Pascals principle? A Lift pump B Hydrometer C Bunsen burner D Hydraulic Jack
30
Gas pressure in container is due to A momentum of the gas particles B change in the momentum of the gas particles C rate of change in the momentum of the gas particles. D rate of change in the momentum of the gas particles per unit area.
31
An iron cyclinder containing gas has a pressure of 360 kPa when it is kept in store at temperature 27 0C. Calculate the pressure of the gas when the cyclinder of the gas is removed outdoors with the temperature of 40 0C. A 243.0 kPa B 345.0 kPa C 375.6 kPa D 533.0 kPa
32
Diagram shows a gas molecules inside a cylinder. The piston is moved slowly downwards and the temperature of the gas stays the same.
Why does the pressure of the gas increase? A The molecules collide harder with the walls B The molecules collide more often with the walls C The molecules move more quickly D The number of molecules increases 33 Diagram shows two copper blocks L and M, in contact with each other. The initial temperatures of L and M are 50 C and 30 C respectively.
Which statement is correct when L and M are at thermal equilibrium? A Temperature of L is higher than M B The quantity of heat in L is the same as in M C Rate of change in temperature of L is bigger than that of M D Net rate of heat flow between L and M is zero 34 Before a long journey, the air in a tyre of a lorry has a pressure of 128 kPa and a temperature of 27C. After the journey the air pressure in the tyre is 132 kPa. Which expression determines the temperature of the air in the tyre after the journey? [Assume the volume of the tyre is constant]
35
36
A box of 6 kg mass is being pulled up a ramp by a force F at a constant speed as shown .Given that the friction along the surface is 4N, what is the force F required to bring the box to the top of the ramp?(g = 10 N kg-1) Fig: A. 4 N B. 40 N C. 44 N D. 64 N In the Brownian motion experiment, what happens when the specimen is being heated ? A B The air particles move faster and bombard the smoke particles more rapidly.
Вам также может понравиться
- Physics Y10 Term 2 Exam Paper1 EditedДокумент10 страницPhysics Y10 Term 2 Exam Paper1 EditedEzra Loganathan MuniandiОценок пока нет
- Physics Y10 Term 2 Exam Paper1 EditedДокумент10 страницPhysics Y10 Term 2 Exam Paper1 EditedUswa ChОценок пока нет
- Kinetic Molecular Theory ExercisesДокумент16 страницKinetic Molecular Theory Exercisesmaatla monkgeОценок пока нет
- Modul 2Документ7 страницModul 2Goh Ling YuehОценок пока нет
- Physics Paper 1 Form 4Документ21 страницаPhysics Paper 1 Form 4Salmizam Izam0% (1)
- Pressure Questions For IGCSE PhysicsДокумент14 страницPressure Questions For IGCSE PhysicsLai Kee Kong100% (1)
- Pressure Questions For IGCSE PhysicsДокумент14 страницPressure Questions For IGCSE PhysicsMohamed Jameel77% (31)
- Pressure AnsДокумент2 страницыPressure AnsHansraj RahulОценок пока нет
- Chapter 4: HeatДокумент13 страницChapter 4: HeatUmi Salwa Kamal ArifinОценок пока нет
- Igcse Physics MCДокумент5 страницIgcse Physics MCayeayeОценок пока нет
- KTG & Thermodynamics (QB)Документ16 страницKTG & Thermodynamics (QB)Raju SinghОценок пока нет
- Physic's Paper 1 Form4-08Документ15 страницPhysic's Paper 1 Form4-08Salmizam IzamОценок пока нет
- Soalan Paper 1 Fizik Elasticity - PressureДокумент17 страницSoalan Paper 1 Fizik Elasticity - Pressureimposter131Оценок пока нет
- Worksheet Gas LawДокумент16 страницWorksheet Gas LawMohamad Rizal MukhtarОценок пока нет
- Teori Kinetik GasДокумент20 страницTeori Kinetik GasShintaYuliaОценок пока нет
- Physics Sample Paper For o Levels. Student Can Get Help To Solve o Level PaperДокумент25 страницPhysics Sample Paper For o Levels. Student Can Get Help To Solve o Level Paperrana ALIОценок пока нет
- The New AnswerДокумент11 страницThe New AnswerayydenОценок пока нет
- Chapter Pressure:Lesson 3.2Документ7 страницChapter Pressure:Lesson 3.2Rais RahimiОценок пока нет
- Pressure Questions For IGCSE PhysicsДокумент14 страницPressure Questions For IGCSE PhysicsAgus SetyawanОценок пока нет
- WORK Sheet PressureДокумент6 страницWORK Sheet PressureSherazОценок пока нет
- Sekolah Menengah Kebangsaan Kubong, LimbangДокумент14 страницSekolah Menengah Kebangsaan Kubong, LimbangDuong Han CalebОценок пока нет
- Set 1 Ques. 1 Figure Shows The Reading From A Scale of Vernier CaliperДокумент18 страницSet 1 Ques. 1 Figure Shows The Reading From A Scale of Vernier Caliper林柄洲Оценок пока нет
- KTG and Thermodynamics PDFДокумент15 страницKTG and Thermodynamics PDFmayank singhОценок пока нет
- SPM Physics Summative Test 3 - Form 4 Chapter 3 4 5Документ7 страницSPM Physics Summative Test 3 - Form 4 Chapter 3 4 5Winnie Lim Li SzeОценок пока нет
- KTG & Thermodynamics - BankДокумент15 страницKTG & Thermodynamics - BankSunita MauryaОценок пока нет
- Class 10 p1 FinalДокумент12 страницClass 10 p1 FinalNeural Spark Physics CieОценок пока нет
- KTG & Thermodynamics (QB)Документ18 страницKTG & Thermodynamics (QB)hodeegits9526Оценок пока нет
- LESSON 3.2 Understanding Pressure in Liquids: Example 1Документ4 страницыLESSON 3.2 Understanding Pressure in Liquids: Example 1Joseph TingОценок пока нет
- Tutorial 3.3Документ20 страницTutorial 3.3cikgusuriyatiОценок пока нет
- CPP FiitjeeДокумент18 страницCPP FiitjeeAmey Kale0% (1)
- 9702 p1 Properties of Matter AllДокумент8 страниц9702 p1 Properties of Matter AllNushanKhanОценок пока нет
- Problems - Introduction and Fluid Statics - 1Документ5 страницProblems - Introduction and Fluid Statics - 1Asjad khanОценок пока нет
- Fluid Properties (HW1)Документ4 страницыFluid Properties (HW1)Jamiel CatapangОценок пока нет
- Fluids SubjectiveДокумент11 страницFluids SubjectiveSayantan ChatterjeeОценок пока нет
- Pascal, Des & BernoulliДокумент15 страницPascal, Des & BernoulliNor Hidaya Md SaidОценок пока нет
- NAIS Final Exam 2020-2021 Semester 2: Subject: AP PHYSICS 2Документ9 страницNAIS Final Exam 2020-2021 Semester 2: Subject: AP PHYSICS 2Horacio FerrándizОценок пока нет
- Tutorial 3.2Документ22 страницыTutorial 3.2cikgusuriyatiОценок пока нет
- 3.2 Liquid Pressure 2012Документ12 страниц3.2 Liquid Pressure 2012cikgusuriyati100% (1)
- ExercisesДокумент19 страницExercisesNhật MinhОценок пока нет
- FZK Upp2 2011Документ16 страницFZK Upp2 2011Amalina KamarudinОценок пока нет
- PAPER-1 Mock Paper 2015Документ10 страницPAPER-1 Mock Paper 2015Ishtiyaq AhmedОценок пока нет
- Contoh Soalan Program Puncak Usaha SPM 2011 (Fizik)Документ31 страницаContoh Soalan Program Puncak Usaha SPM 2011 (Fizik)kentchuanОценок пока нет
- Level 3 6090-30 502 ST Ver 1.0 Sep 2017Документ19 страницLevel 3 6090-30 502 ST Ver 1.0 Sep 2017e4erkОценок пока нет
- SPM 2005 Paper 1 + SkimaДокумент26 страницSPM 2005 Paper 1 + SkimaZabri Mat BasirОценок пока нет
- Fluid Mechanics PDFДокумент6 страницFluid Mechanics PDFmohapatramugdha99Оценок пока нет
- Homework SolutionДокумент25 страницHomework SolutionHirman De NovaОценок пока нет
- Hydrostatics PDFДокумент13 страницHydrostatics PDFNsBhasinОценок пока нет
- FizikДокумент36 страницFizikLileng LeeОценок пока нет
- Tutorial 3.3: Gas & Atmospheric PressureДокумент20 страницTutorial 3.3: Gas & Atmospheric PressurePauling ChiaОценок пока нет
- Supplement Questions of FLUID MECHANICS: Paragraph For Question Nos. 1 To 3Документ7 страницSupplement Questions of FLUID MECHANICS: Paragraph For Question Nos. 1 To 3Tanuj Gupta0% (2)
- Assignment 1Документ3 страницыAssignment 1Marsha WongОценок пока нет
- IGCSE 2.0 - Thermal Physics - Test 2018Документ9 страницIGCSE 2.0 - Thermal Physics - Test 2018Brandeice BarrettОценок пока нет
- Prac Prob HGE 2Документ8 страницPrac Prob HGE 2jeannechoiashОценок пока нет
- Questionnaire For Elimination RoundДокумент7 страницQuestionnaire For Elimination RoundJV CustodioОценок пока нет
- Physical ChemistryДокумент5 страницPhysical ChemistryFran LeeОценок пока нет
- Form 4 Ujian OgosДокумент3 страницыForm 4 Ujian Ogosjesunathan44@yahoo.comОценок пока нет
- SPM 4531 2006 Physics p1 BerjawapanДокумент12 страницSPM 4531 2006 Physics p1 Berjawapanpss smk selandarОценок пока нет
- JMC Pre Mock Light & Wave p1 & p2 QPДокумент10 страницJMC Pre Mock Light & Wave p1 & p2 QPNeural Spark Physics CieОценок пока нет
- Physics 2nd Paper Practical 1Документ1 страницаPhysics 2nd Paper Practical 1Neural Spark Physics CieОценок пока нет
- TBRPhysics2 PDFДокумент308 страницTBRPhysics2 PDFNeural Spark Physics CieОценок пока нет
- 11 Physics Revision Book Solutions 1 PDFДокумент14 страниц11 Physics Revision Book Solutions 1 PDFNeural Spark Physics CieОценок пока нет
- CH 5Документ25 страницCH 5anil.gelra5140Оценок пока нет
- FogДокумент307 страницFogmshamil100% (1)
- FAA P 8740 02 DensityAltitude (Hi Res) BrandedДокумент8 страницFAA P 8740 02 DensityAltitude (Hi Res) BrandedrayduxОценок пока нет
- 4 Types of Climate in The PhilippinesДокумент3 страницы4 Types of Climate in The PhilippinesDA CVRCОценок пока нет
- Case StudyДокумент5 страницCase StudyAnn De Vera100% (1)
- Gr8Q2W4 ScienceДокумент17 страницGr8Q2W4 ScienceMaylyn Grace Dalumpines-Colon EbonaloОценок пока нет
- Hydrology NotesДокумент164 страницыHydrology Notespraneetha reddy100% (1)
- Climatic Data MeasurementДокумент30 страницClimatic Data MeasurementchandanaОценок пока нет
- Method For Calculating OPGW Optical Cable Short Circuit Current Heat Effect by Using Improved Synthetic MethodДокумент25 страницMethod For Calculating OPGW Optical Cable Short Circuit Current Heat Effect by Using Improved Synthetic MethodHugh cabОценок пока нет
- Garbage DumpingДокумент48 страницGarbage DumpingRashmeetОценок пока нет
- Biol448 L2Документ57 страницBiol448 L2shimaa aldamenОценок пока нет
- Env55 Metnh3 Metrology For Ammonia in Ambient Air" 2 Metnh3 Workshop On Ammonia Measurement MethodologyДокумент25 страницEnv55 Metnh3 Metrology For Ammonia in Ambient Air" 2 Metnh3 Workshop On Ammonia Measurement Methodologylaoying qdОценок пока нет
- Lesson Plan-Otbaclean Air-A Shared Concern 2Документ9 страницLesson Plan-Otbaclean Air-A Shared Concern 2api-53533418Оценок пока нет
- Edexcel Module Heat Transfer and Combustion - H2 Outcome 4 - Tutorial 1Документ20 страницEdexcel Module Heat Transfer and Combustion - H2 Outcome 4 - Tutorial 1gems_gce074325Оценок пока нет
- Climate For StudentsДокумент7 страницClimate For StudentsDelia MihalcaОценок пока нет
- Gen Ed Science ReviewerДокумент4 страницыGen Ed Science ReviewerZoila MiraОценок пока нет
- ASSIGNMENT #1 Environmental ScienceДокумент14 страницASSIGNMENT #1 Environmental ScienceSyed Khurram ShahzadОценок пока нет
- Teach With Space: The Greenhouse Effect and Its ConsequencesДокумент27 страницTeach With Space: The Greenhouse Effect and Its ConsequencesAna BritoОценок пока нет
- Environment Current Affairs 2020Документ60 страницEnvironment Current Affairs 2020ShahbazAkramОценок пока нет
- Global Warming and Climate ChangeДокумент2 страницыGlobal Warming and Climate ChangeHou TaroОценок пока нет
- What Keeps Us and Other Organisms AliveДокумент19 страницWhat Keeps Us and Other Organisms AliveSharmen QuijoteОценок пока нет
- Lecture 6 ExamplesДокумент29 страницLecture 6 ExamplesMohamed ZahranОценок пока нет
- F3 Chapter 9 Space WeatherДокумент9 страницF3 Chapter 9 Space WeatherJue Hazea GoldshopОценок пока нет
- 200 More Puzzling Physics-AprofundamentoДокумент21 страница200 More Puzzling Physics-AprofundamentoPaulo CesarОценок пока нет
- Density of AirДокумент3 страницыDensity of AirAurea Jasmine DacuycuyОценок пока нет
- Energies: Numerical Simulations On The Application of A Closed-Loop Lake Water Heat Pump System in The Lake Soyang, KoreaДокумент16 страницEnergies: Numerical Simulations On The Application of A Closed-Loop Lake Water Heat Pump System in The Lake Soyang, KoreaMvikeli DlaminiОценок пока нет
- TP Phy101Документ11 страницTP Phy101Keisham Nabachandra SinghaОценок пока нет
- Factors Affecting ThrustДокумент4 страницыFactors Affecting ThrustShabeer830100% (2)
- Earth Science Study Guide Answers FinalДокумент73 страницыEarth Science Study Guide Answers FinalIAN GamingОценок пока нет
- Answers Should Be Written in QCAB Format Only.: InstructionsДокумент76 страницAnswers Should Be Written in QCAB Format Only.: InstructionsSandeep PrajapatiОценок пока нет
- Psych Rome TryДокумент21 страницаPsych Rome TryEst LijОценок пока нет