Академический Документы
Профессиональный Документы
Культура Документы
Percent of Oxygen in Air
Загружено:
pankajbtc007Исходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Percent of Oxygen in Air
Загружено:
pankajbtc007Авторское право:
Доступные форматы
Percent Oxygen in Air
Trap some gas Figure out the percent of oxygen in the atmosphere by using steel wools ability to rust. Materials: Test tube- standard size (actually any container will work) Clear glass of water Soap or vinegar Water Graduated cylinder Fine mesh steel wool - 00 or finer Tape or marker pen To do and notice: 1. Tear of a small amount of steel wool (less than a gram will work fine) and push it to the bottom of a test tube. 2. The steel wool must be wedged into the base. Push it in hard enough so that it will not slide out when the test tube is flipped upside down. 3. Measure the volume of the test tube. This can be done with a graduated cylinder and some water (You are measuring the volume of how much water it can hold. This in turn, measures the amount of air in the empty test tube (There is so little steel wool, its volume is negligible). 4. Enter this data in the worksheet. 5. Your steel wool probably got wet when measuring volume in step 3. However, to insure you got rid of the thin protective oil coating on the steel wool, rinse your test tube out with either soapy water or vinegar. This will help to facilitate the rusting of the steel wool. 6. Fill a clear cup about half way with water. 7. Place the empty test tube (with steel wool inside) upside in the water. 8. Place your experiment in a calm protected spot for at least 2 days. After at least 2 days: 9. Notice that the water level has risen inside the test tube. 10. Without letting the water leak out of the inverted test tube, mark the water level on the outside of the tube with a pen or piece of tape. Eric Muller - copyright 2000 (original document 1988)
11. Measure the volume of gas in the tube now. You can do this by taking the test tube out of the glass (The water inside the test tube can leak out at this point). Fill the test tube up to the marking from step 9. Now, measure this amount of water. 12. Enter this data on the worksheet. 13. Figure out the percent change in gas in the test tube (see worksheet). 14. This volume change is the percent Oxygen that was in the test tube. 15. Collect data from as many experiments as possible and average the percentage. Whats going on? Air is composed of Nitrogen - about 78%, Oxygen about 21%, Argon about 1% and other gasses. These gasses compose 100% of the air so they exert 100% of the pressure. IN the test tube, at the beginning, there was the same pressure inside the test tube as outside. As the steel wool started to rust*, the oxygen was taken out of the air, therefore the pressure was reduced. Over time, the pressure was reduced by the percentage of oxygen removed. about 21%. As the pressure drops, the pressure on the outside of the test tube pushes the water level up inside the test tube. *The iron in the steel wool became an iron oxide. The darker steel wool probably became Fe3O4 or Fe2O3. The orange colored steel wool probably became FeO[OH]
Eric Muller - copyright 2000 (original document 1988)
Percent of Oxygen in Air Work Sheet
_______________________________________________
A. Volume of empty test tube (Vempty) = ________ml B. Volume of test tube after rusting (Vrust) = ______ml C. Percent of Oxygen in Air (in 2 steps): 1. Percent of remaining gas (Premain) = ____ml (Vrust) / _____ml(Vempty) x 100 = ____% 2. Percent of Oxygen in Air = 100 ______ %(Premain) = ________%
D. Class Average of the percent of Oxygen in the Air = ________%
Eric Muller - copyright 2000 (original document 1988)
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Sabre-Baggage Management and Analysis SystemДокумент24 страницыSabre-Baggage Management and Analysis Systemdifaini anugrahОценок пока нет
- Epson Eb-W22 ProjectorДокумент2 страницыEpson Eb-W22 Projectorandresboy123Оценок пока нет
- General Construction Notes: FC Engineering ServicesДокумент1 страницаGeneral Construction Notes: FC Engineering ServicesMac KYОценок пока нет
- Photosynthesis LabДокумент3 страницыPhotosynthesis Labapi-276121304Оценок пока нет
- Quidway 20S5300 20series 20ethernet 20switches 20hardware 20Документ98 страницQuidway 20S5300 20series 20ethernet 20switches 20hardware 20Hamza_yakan967Оценок пока нет
- Pre-Spud Checklist # 4Документ2 страницыPre-Spud Checklist # 4Yougchu LuanОценок пока нет
- Ruckus Wired Accreditation ExamДокумент15 страницRuckus Wired Accreditation ExamDennis Dube25% (8)
- Alloc 150 DMДокумент301 страницаAlloc 150 DMSheik Mohamed ImranОценок пока нет
- DRM Transmitter PresentationДокумент22 страницыDRM Transmitter PresentationJuan Jose PerezОценок пока нет
- CST 336 Final Project Computown DocumentationДокумент12 страницCST 336 Final Project Computown Documentationapi-461214598Оценок пока нет
- Bell Desk-2Документ96 страницBell Desk-2Arrow PrasadОценок пока нет
- 3 3 1 Material Sorter Design ChallengeДокумент3 страницы3 3 1 Material Sorter Design Challengeapi-343534512Оценок пока нет
- Isuzu 4hk1x Sheet HRДокумент4 страницыIsuzu 4hk1x Sheet HRMuhammad Haqi Priyono100% (1)
- MMD 74 XX DR PS 0020 - C03Документ1 страницаMMD 74 XX DR PS 0020 - C03bramexОценок пока нет
- Strength of Materials Basics and Equations - Mechanics of Materials - Engineers EdgeДокумент6 страницStrength of Materials Basics and Equations - Mechanics of Materials - Engineers EdgeansarОценок пока нет
- ICON Catalog LocationsДокумент16 страницICON Catalog LocationsTools StuffsОценок пока нет
- MOTOR Brushless ss2814 Xiii 1000kvДокумент1 страницаMOTOR Brushless ss2814 Xiii 1000kvsalah eddineОценок пока нет
- Vertical Take Off and LandingДокумент126 страницVertical Take Off and LandingMukesh JindalОценок пока нет
- (Ebook - Electronics) - Principles of PLL - Tutorial (Kroupa 2000)Документ66 страниц(Ebook - Electronics) - Principles of PLL - Tutorial (Kroupa 2000)양종렬Оценок пока нет
- Electrical SubstationsДокумент16 страницElectrical SubstationsEngr Syed Numan ShahОценок пока нет
- Treatment Processes: Coagulation and Filtration: Draft Guidelines For Drinking-Water QualityДокумент25 страницTreatment Processes: Coagulation and Filtration: Draft Guidelines For Drinking-Water QualityAbsharinaОценок пока нет
- 02-Dr Ooi-Design of Jacked-In Piles & Case Studies in SingaporeДокумент39 страниц02-Dr Ooi-Design of Jacked-In Piles & Case Studies in SingaporefreezefreezeОценок пока нет
- Detailed Lesson Plan in Science 5Документ5 страницDetailed Lesson Plan in Science 5hs4fptm82gОценок пока нет
- Desmophen 1200 - en - 00134597 17947398 20766463Документ3 страницыDesmophen 1200 - en - 00134597 17947398 20766463Sabri AeroChemОценок пока нет
- Hyundai Robex 220LC-9A SpecificationsДокумент14 страницHyundai Robex 220LC-9A SpecificationsKundan DhurveОценок пока нет
- 4 MPM Scope - OutputДокумент45 страниц4 MPM Scope - OutputSajid Ali MaariОценок пока нет
- Project Based Lab Report ON Voting Information System: K L UniversityДокумент13 страницProject Based Lab Report ON Voting Information System: K L UniversitySai Gargeya100% (1)
- Department of Education: Republic of The PhilippinesДокумент14 страницDepartment of Education: Republic of The PhilippinesRich TactaconОценок пока нет
- Parth Valves and Hoses LLP.: Test & Guarantee CertificateДокумент1 страницаParth Valves and Hoses LLP.: Test & Guarantee CertificateSURYAKANTОценок пока нет
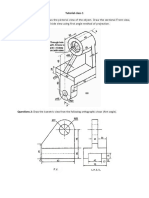
- Tutorial Class 1 Questions 1Документ2 страницыTutorial Class 1 Questions 1Bố Quỳnh ChiОценок пока нет