Академический Документы
Профессиональный Документы
Культура Документы
Ejercicios de termodinámica y problemas resueltos
Загружено:
650301Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Ejercicios de termodinámica y problemas resueltos
Загружено:
650301Авторское право:
Доступные форматы
EJERCICIOS DE TERMODINAMICA Problemas Captulo 1. 1. Calculate the work performed by a body expanding from an initial volume of 3.
12 liters to a final volume of 4.01 liters at the pressure of 2.34 atmospheres. Se sustituyen los datos en la integral para un cambio finito de volumen de V1 a V2 W= pdV Se obtiene el trabajo efectuado en un cambio de volumen a presin constante. W= p (V2-V1) W= 2.34 atm (4.01 lt- 3.12 lt) W= (237100.5 Kg/ms) (0.00401m-.00312m) W= 211.01 Kg/mS. m W=211.01 Kgm/ s = Energa (la p y el V son 2 variables cannicamente conjugadas)
2. Calculate the pressure of 30 grams of hydrogen inside a container of 1 cubic meter at the temperature of 18C Sustituimos los valores en la ecuacin de los gases ideales teniendo la constante de los gases R= 8314472 J/mol.K
pV= m/M (RT) p=m RT/V p= 30 moles/ 1m (8314472 J/mol.K x 291 K) p= 7.25 x 10 J/m p= 7.25 x10 Kg/ ms
3. Calculate the density and specific volume of nitrogen at the temperature of 0C
4. Calculate the work performed by 10 grams of oxygen expanding isothermally at 20C from 1 to .3 atmospheres of pressure De la ecuacin del trabajo efectuado en un cambio de volumen sustituimos la presin de n moles de gas ideal. W= pdV donde p= nRT Sustituimos esto en la integral, y sacamos las constantes n, R y T y calculamos la integral: W= n RT dV/V = nRT ln V2/V1 Cuando T es constant p1V1= p2V2 o bien V2/V1=p/P2 Asi que el trabajo isotrmico tambin puede expresarse como: W= nRT ln p2/p1 (gas ideal, proceso isotrmico) W= 10 mol (8314472 J/mol.K) (273 K) Ln(303975 Kg/ms/101325 Kg/ms) W= 1.77x10 Ln 3 W=1.9293x10 Kgm/ s = energia
Problemas capitulo 2. 1. Calculate the energy variation of a sistem which performs 3.4 X 10 ergs of work and absorbs 32 calories of heat.
2. How many calories are absorbed by 3 moles of an ideal gas expanding isothermally from the initial pressure of 5 atmospheres to the final pressure of 3 atmospheres, at the temperature of 0C?
3. One mole of a diatomic ideal gas performs a transformation from an initial state for which temperature and volume are, respectively, 291K and 21000 cc. to a final state in which temperature and volume are 305K and 12700 cc. The transformation is represented on the (V,p) diagram by a straight line. To find the work performed and the heat absorbed by the system. 4. 4. A diatomic gas expands adiabatically to a volume 1.35 times larger than the initial volume. The initial temperature is 18C. Find the final temperature.
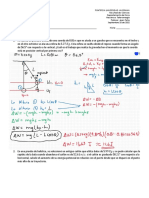
Problemas Capitulo 3. 1. One mole of a monatomic gas performs a Carnot cycle between the temperaturas 400K and 300K. On the upper isothermal transformation, the initial volume is 1 liter and the final volume 5 liters. To find the work performed during a cycle, and the amounts of heat exchanged with the two sources.
2. What is the maximum efficiency of a thermal engine working between an upper temperature of 400C and a lower temperature of 18C?
3. Find the minimum amount of work needed to extract one calorie of heat from a body at the temperature of 0F, when the temperature of the environment is 100F.
Problemas Capitulo 4. 1. What is the entropy variation of 1000 grams of water when raised from freezing to boiling temperature? (assume a constant specific heat= 1 cal/gm.deg)
2. A body obeys the equation of state:
pV= 10T A measurement of its thermal capacity inside a container having the constant volume 100 liters shows that under these conditions, the thermal capacity is constant and equal to 0.1 cal/deg. Express the energy and the entropy of the system as functions of T and V. 3. The boiling point of ethyl alcohol (CHO) is 78.3C; the heat of vaporization is 855 Joules/gm. Find dp/dT at the boiling point.
Problemas capitulo 5. 1. With the aid of the phase rule discuss the equilibrium of a saturated solution and the solid of the dissolved substance.
2. How many degrees of freedom has the system composed of a certain amount of water and a certain amount of air? (Neglect the rare gases and the carbon dioxide contained in air)
3. The electromotive force of a reversible electric cell, as a function of the temperature, is: 0.924 + 0.0015t + 0.0000061t volts t being the temperature in C. Find the heat absorbed by the cell when one coulomb of electricity flows through it isothermally at a temperature of 18C.
Вам также может понравиться
- Termodinamica Fase 4Документ5 страницTermodinamica Fase 4luis krlos hernxndzОценок пока нет
- TERMOQUIMICAДокумент2 страницыTERMOQUIMICAMELANY MIRELLA FLORES RODRIGUEZОценок пока нет
- Problemas Resueltos 4Документ24 страницыProblemas Resueltos 4Yerson Alcedo Espinoza33% (6)
- Práctica 3er ParcialДокумент2 страницыPráctica 3er ParcialhelenОценок пока нет
- Entropía y cambios de estado en sistemas termodinámicosДокумент4 страницыEntropía y cambios de estado en sistemas termodinámicosJose Vásquez GómezОценок пока нет
- Preguntas Resueltas FisicaДокумент4 страницыPreguntas Resueltas FisicaLincoln GarciaОценок пока нет
- Cálculos de rendimiento y cambios de entropía para procesos termodinámicosДокумент6 страницCálculos de rendimiento y cambios de entropía para procesos termodinámicosDaniel NoreñaОценок пока нет
- Examen Parcial de Termodinamica para La Ingenieria IДокумент14 страницExamen Parcial de Termodinamica para La Ingenieria IMauricio RamirezОценок пока нет
- Tarea Semana 3 TermodinamicaДокумент6 страницTarea Semana 3 TermodinamicaGerman Morera Bedoya100% (2)
- Practica #3 Termodinamica LlenaДокумент7 страницPractica #3 Termodinamica LlenaHANNEL MELO100% (3)
- Termodinamica Semana 3 MejДокумент11 страницTermodinamica Semana 3 MejMatías De León Jimenez100% (2)
- Ejercicios Propuestos de TermodinamicaДокумент4 страницыEjercicios Propuestos de TermodinamicaRuth Rojas Ramirez0% (1)
- Guia de SA y SC 2021 - F - Q para EntregarДокумент7 страницGuia de SA y SC 2021 - F - Q para EntregarLeandro PonceОценок пока нет
- ACT 4 Termodinamica Grupo#201015 49Документ31 страницаACT 4 Termodinamica Grupo#201015 49Helen AyalafajardoОценок пока нет
- Documento de Fiama SalcedoДокумент13 страницDocumento de Fiama SalcedoFiama Salcedo MedinaОценок пока нет
- PRÁCTICA DE FISICOQUÍMICA GASES Y 1a LEY TERMODINÁMICAДокумент4 страницыPRÁCTICA DE FISICOQUÍMICA GASES Y 1a LEY TERMODINÁMICAerizoОценок пока нет
- Tarea S4 Termo/iaccДокумент11 страницTarea S4 Termo/iaccSebastian Molina100% (8)
- Angel Aguirre Tarea 4Документ7 страницAngel Aguirre Tarea 4Angel Aguirre CarrascoОценок пока нет
- Trabajo Práctico #5Документ4 страницыTrabajo Práctico #5Carli CastilloОценок пока нет
- Problemario Termo 2Документ4 страницыProblemario Termo 2Hector SustaitaОценок пока нет
- Cálculos de potencia de turbina y cambios de volumen y entropía en procesos termodinámicosДокумент4 страницыCálculos de potencia de turbina y cambios de volumen y entropía en procesos termodinámicosKarín AmadoОценок пока нет
- Máquinas Térmicas - Problemas de sustancias puras y mezclas bifásicasДокумент31 страницаMáquinas Térmicas - Problemas de sustancias puras y mezclas bifásicasChristian Ticona Zambrano100% (2)
- Turbina de gas adiabática expande aire a 850 kPa y 420°CДокумент6 страницTurbina de gas adiabática expande aire a 850 kPa y 420°CJosdiaAlvarbelaezОценок пока нет
- Practica 02 - Termodinamica 02 - 2013Документ8 страницPractica 02 - Termodinamica 02 - 2013Anonymous malHQ6Оценок пока нет
- ejercicios quimicaДокумент6 страницejercicios quimicaHADESОценок пока нет
- Problemas Resueltos Tema 3-Segunda LeyДокумент45 страницProblemas Resueltos Tema 3-Segunda LeyISMAELCOLQUE100% (3)
- Ejercicio 3Документ22 страницыEjercicio 3Carlos Raul67% (3)
- Trabajo Práctico #6 Transformaciones Energéticas-1Документ3 страницыTrabajo Práctico #6 Transformaciones Energéticas-1jorge veraОценок пока нет
- Calcule El Calor Necesario para Calentar 200Kg de N2O de 20Документ2 страницыCalcule El Calor Necesario para Calentar 200Kg de N2O de 20GABRIELAОценок пока нет
- Ejercicios Grupo 1 - 6Документ9 страницEjercicios Grupo 1 - 6Douglas SchmidtОценок пока нет
- Trabajo Práctico #6 Transformaciones EnergéticasДокумент3 страницыTrabajo Práctico #6 Transformaciones EnergéticasFabian GimenezОценок пока нет
- TallerДокумент4 страницыTallerYireth Beleño OrtizОценок пока нет
- ENTROPÍAДокумент40 страницENTROPÍAALEXIS ENEQUE FLORESОценок пока нет
- ENERGIAUTILIZABLEДокумент10 страницENERGIAUTILIZABLEEduardo ContentoОценок пока нет
- S10.s1 - Ejercicios para Resolver - TermidinámicaДокумент2 страницыS10.s1 - Ejercicios para Resolver - TermidinámicaJimena LimacheОценок пока нет
- Problemas de La 2 Ley de La TermodinámicaДокумент11 страницProblemas de La 2 Ley de La Termodinámicanaleny ruiz cruzОценок пока нет
- Ejercicios Propuestos de 1° Ley TermodinamicaДокумент7 страницEjercicios Propuestos de 1° Ley TermodinamicaRAQUEL NANCY VELIZ SAGARVINAGA0% (1)
- TPS2 234 12108219 20231Документ12 страницTPS2 234 12108219 20231INVERSIONES AMBIENTALES MLNS C.A.Оценок пока нет
- PROBLEMAS TermodinamicaДокумент31 страницаPROBLEMAS TermodinamicaJulio Alberto Durand AñazcoОценок пока нет
- Ejercicios Segunda Ley 2020-IIДокумент2 страницыEjercicios Segunda Ley 2020-IIStefany Elizabeth Crisostomo QuispeОценок пока нет
- Ejercicios Segunda Ley 2020-IIДокумент2 страницыEjercicios Segunda Ley 2020-IIStefany Elizabeth Crisostomo QuispeОценок пока нет
- Taller 2 Química Física.........................Документ12 страницTaller 2 Química Física.........................OM TfaОценок пока нет
- EP - Tema 3. Termodinámica - Ejercicios Propuestos Parte 1 RESUELTO UNIVERSIDADДокумент7 страницEP - Tema 3. Termodinámica - Ejercicios Propuestos Parte 1 RESUELTO UNIVERSIDADJuan FernándezОценок пока нет
- Taller 5. Primera Ley de La Termodinámica-1Документ3 страницыTaller 5. Primera Ley de La Termodinámica-1ABIGAIL CRUZОценок пока нет
- Transferencia de calor y primer principio de la termodinámicaДокумент17 страницTransferencia de calor y primer principio de la termodinámicaDavid herreraОценок пока нет
- Ejercicios IndividualesTERMODINAMICAДокумент8 страницEjercicios IndividualesTERMODINAMICAGleydisОценок пока нет
- 2da Ley ENTROPIAДокумент3 страницы2da Ley ENTROPIAStefany Elizabeth Crisostomo QuispeОценок пока нет
- Fase 3 Yesica MendozaДокумент13 страницFase 3 Yesica Mendozayesica mendozaОценок пока нет
- Termodinámica y calorimetríaДокумент49 страницTermodinámica y calorimetríaAndrés Inzunza JaramilloОценок пока нет
- Entrop T06Документ6 страницEntrop T06Juan Gonzalo Rose0% (1)
- Ejercicios Primera Ley de La TermodinamicaДокумент4 страницыEjercicios Primera Ley de La TermodinamicaCamilo Buitrago0% (1)
- Desarrollar y presentar primera fase situación problemaДокумент7 страницDesarrollar y presentar primera fase situación problemaMauricio SuarezОценок пока нет
- Problemas4 TermoДокумент3 страницыProblemas4 TermomgobellanОценок пока нет
- IQM 223. Ejercicios. Serie 1 II 2021Документ2 страницыIQM 223. Ejercicios. Serie 1 II 2021Daniela Alejandra SuarezОценок пока нет
- Ejercicios CORREJIDOS lUIS CARRERAДокумент14 страницEjercicios CORREJIDOS lUIS CARRERADaniel Jose Ortiz LopezОценок пока нет
- T Gui A de Problemas para Taller Previo PC1 FQДокумент5 страницT Gui A de Problemas para Taller Previo PC1 FQNavarro Saavedra Dayanna RusbelithОценок пока нет
- Ingeniería química. Soluciones a los problemas del tomo IОт EverandIngeniería química. Soluciones a los problemas del tomo IОценок пока нет
- Interesa A La NASA Henequén YucatecoДокумент2 страницыInteresa A La NASA Henequén Yucateco650301Оценок пока нет
- Pato de Jottabich o Pato BebedorДокумент2 страницыPato de Jottabich o Pato Bebedor650301Оценок пока нет
- Cálculo del módulo y ángulos de vectores en el espacio tridimensionalДокумент9 страницCálculo del módulo y ángulos de vectores en el espacio tridimensional650301Оценок пока нет
- Norma BolsareutilizableДокумент15 страницNorma Bolsareutilizable650301Оценок пока нет
- NMX C 359 1988Документ26 страницNMX C 359 1988650301Оценок пока нет
- Curvas de titulación de aminoácidosДокумент3 страницыCurvas de titulación de aminoácidosMarioAlfredoMtzОценок пока нет
- Trabajo Del ClimaДокумент13 страницTrabajo Del ClimaAriana ViОценок пока нет
- Taller Energía y SoluciónДокумент7 страницTaller Energía y Soluciónfotosyvideo SharedОценок пока нет
- Análisis Vectorial I Jueves 18Документ2 страницыAnálisis Vectorial I Jueves 18Said ZuñigaОценок пока нет
- Planta de AmoniacoДокумент20 страницPlanta de AmoniacoLuis Alberto Miranda TerrazasОценок пока нет
- Tarea Preparatoria Gases, Compuertas y EmpujeДокумент7 страницTarea Preparatoria Gases, Compuertas y EmpujeDiego AlonzoОценок пока нет
- Determinacion Experimental Del Equilibrio Liquido VaporДокумент5 страницDeterminacion Experimental Del Equilibrio Liquido VaporCatherine CentanaroОценок пока нет
- Teoría Máquinas MecanismosДокумент2 страницыTeoría Máquinas MecanismosLuis MejiaОценок пока нет
- VpoyntingДокумент7 страницVpoyntingMiguel Tasambay SalazarОценок пока нет
- Ejercicio 9.3 RepresentacionДокумент11 страницEjercicio 9.3 RepresentacionMariela Mejía100% (1)
- Reflectores AcusticosДокумент3 страницыReflectores AcusticosFray Carranza MestanzaОценок пока нет
- Correlaciones para El Calculo Del H ConvectivoДокумент11 страницCorrelaciones para El Calculo Del H ConvectivoLuis B VasquezОценок пока нет
- TP Nº2 Equilibrio Químico - InformeДокумент6 страницTP Nº2 Equilibrio Químico - InformeInsy HОценок пока нет
- Membranas para purificación de aguaДокумент12 страницMembranas para purificación de aguaGvrОценок пока нет
- Evaluacion Quimica Teorias Atomos IonesДокумент4 страницыEvaluacion Quimica Teorias Atomos IonesVictoria Alejandra FsmОценок пока нет
- Tema3 EnlacequimicoДокумент6 страницTema3 EnlacequimicoClari pОценок пока нет
- CALCULOДокумент28 страницCALCULODante Nuñez OcsaОценок пока нет
- Separación de partículas de sílice y galenaДокумент8 страницSeparación de partículas de sílice y galenaCriz Espinoza ChavezОценок пока нет
- Generalidades de Transferencia de CalorДокумент4 страницыGeneralidades de Transferencia de CalorKatia Ruelas TorresОценок пока нет
- Unidad 3 Migracion de HidrocarburosДокумент22 страницыUnidad 3 Migracion de HidrocarburosYimar MОценок пока нет
- Enfriador MostoДокумент2 страницыEnfriador MostoAndrea Del Pilar ObesoTantapomaОценок пока нет
- Afiche Soldadora Tig V1Документ1 страницаAfiche Soldadora Tig V1rodrigo zamora tapiaОценок пока нет
- Ud 2. Recurso 4. Colisiones en 2 DimensionesДокумент14 страницUd 2. Recurso 4. Colisiones en 2 DimensionesGeuris Miguel AraujoОценок пока нет
- CromatografíaДокумент85 страницCromatografíaEnrique Velasquez FelipeОценок пока нет
- Parcial3GATerm28junio 761439651Документ2 страницыParcial3GATerm28junio 761439651Benjamín Medina CarrilloОценок пока нет
- Informe Fisica - Amortiguador AntisismicoДокумент17 страницInforme Fisica - Amortiguador AntisismicoKevin GTОценок пока нет
- MetalografiaДокумент4 страницыMetalografiaYahir FelicianoОценок пока нет
- Diseño de Sistema de Agua Potable PDFДокумент6 страницDiseño de Sistema de Agua Potable PDFNick FlОценок пока нет
- Teoría de La Relatividad GeneralДокумент3 страницыTeoría de La Relatividad GeneralSamuel ArangoОценок пока нет
- Ejemplo Memoria de Calculo de Fundación de ColectorДокумент16 страницEjemplo Memoria de Calculo de Fundación de ColectorTAEZA SRLОценок пока нет