Академический Документы
Профессиональный Документы
Культура Документы
Thermochemistry
Загружено:
Drazelle PangilinanИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Thermochemistry
Загружено:
Drazelle PangilinanАвторское право:
Доступные форматы
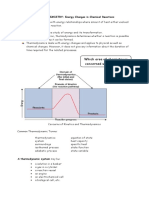
Energy, Enthalpy and Thermochemistry Energy and the First Law of Thermodynamics To begin the study of the transformation
of energy in chemical (or other) processes, we need to first develop a few terms that we will use. These definitions must be very tightly specified and understood in order to properly follow some of the more complex logic in later pages. Definitions: Thermodynamics The science of transformation of energy. Energy The capacity to do work or to transfer heat Work Work has several aspects to it. There is mechanical work, electrical work, light-energy work and work of expansion or contraction of a gas (PV work). One of the most common definitions for work is when a force f displaces an object by a distance x does work w' = f*x. This is a mechanical work. Kinetic and Potential Energy KE = 1/2 mv2 both of these can be forms of work and of heat energy PE = mgh lifting mass m by height h against acceleration due to gravity g = fd Work is organized energy, lifting a book, etc. Heat (defined later) is random (disorganized) energy heat released, sound dissipates through air, when book drops and hits the floor. One can also transfer energy into or out of a sample via light-energy and by electrical work (We include this type of work in w'). In first-year chemistry, we'll not often be interested in these terms directly. For the most part, therefore, we'll set w' to be zero. Work can also be done by pushing back the atmosphere (piston in a cylinder). In this case, one pushes back against the atmospheric pressure Patm and moves the piston a distance x. The force needed to push back the atmosphere is actually PatmA where A is the area of the piston. Hence, we have w = Patm A x = Patm V. We call this atmospheric work. (Note that this has the same units as the previous definition of work). In chemical situations, we are normally interested only in the atmospheric work done when gases are evolved or used up in the reaction. SI units: w has units of Nm = kgm2s-2 = Joules (J). Heat Energy transfer as a result of a temperature differential. First Law of Thermodynamics: Often called the Law of conservation of energy: Energy can neither be created nor destroyed but only changed from one form to another. OR The energy of the universe is constant OR The energy of a system which is isolated from its surroundings is constant. force times distance.
More definitions
System whatever we are interested in: Chemicals in a reaction chemical, reaction mixture, container etc. Surroundings the rest of the universe Closed System Heat may transfer but not matter. Isolated system No heat or matter can transfer between the system and its surroundings. Energy transfer can be done in one of two ways: work w can be done on the system by the surroundings (or vice versa). It can take the form of mechanical work or of electrical energy transfer. Heat q flows from the system to the surroundings (or vice versa) There is a general sign convention that chemists use when describing q and w. When energy flows into the system as a result of heat or work, the sign is positive (the system gains energy). When energy flows out of a system, the sign is negative (the system looses energy. Both q and w refer, not to an amount of energy, but to an amount of energy transferred as a result of the process. Enthalpy For the initial part of this chapter, we will be looking at a specific type of cases where the system undergoing the change is held at constant pressure. This, in fact, represents the way the majority of reactions are done in our chemical experience. We define a term called enthalpy H is the energy transferred between a system and the surroundings under constant pressure. We cannot measure the absolute At this point, we're looking at a black box. We can't really understand what's inside, we just define terms to try to understand it
We're familiar with state functions. For instance, the distance between Kingston and Toronto can be measured by a "straight" line on the map. It never changes. It is only a enthalpy of a system but we can function of the position of the two cities. However, the measure the change in enthalpy for a distance driven to get from Kingston to Toronto depends on process. If the Initial and final states the route we take. Obviously, some routes are shorter than have enthalpies Hi and Hf, respectively others. So, we cannot use a non-state function (distance then the change in enthalpy for the driven) to measure a state function (distance separating two process can be defined as cities) without some specific restrictions on the measurements. In this case, we would need to drive our H = Hf - Hi. vehicle in a straight line over the surface of the earth, ignoring roads lakes and hills (a "crow" could do it hence the This definition is only good if H is a state function, i.e., the change in enthalpy term "as the crow flies" when referring to distances). depends only on the initial and final states, not on the process itself. If we simply measure the heat evolved (or absorbed) during a process, we will not have a measure of the change in enthalpy since, in general, q is not a state function. It depends on how the process occurs. We need to restrict our process measurements to specific conditions in order to be able to measure enthalpy directly. In this case, if we maintain constant pressure and then simply measure the heat transferred as a result of the process qp we will have a measure of the change in enthalpy. The subscript p refers to the fact that this heat was measured at constant pressure conditions. Thus, we have simply H = qp at constant pressure For chemical reactions we can write H = Hproducts - Hreactants
If H is negative, then net flow of heat from chemical system to surroundings (heat released). Exothermic (EXO sounds like exit, from the Latin: to leave) If H is positive, then net flow of heat from surroundings to chemical system (heat absorbed). Endothermic (ENDO sounds like enter) CH4(g) + 2O2(g) ----> CO2(g) + 2H2O(l) H = -890 kJ/mol (that's per mole of Rxn as written) (EXOTHERMIC) H2(g) + I2(g) ----> 2 HI(g) Heat Capacity One of the easiest ways to measure a heat transfer is to measure a change in temperature and then calculate from that, the heat transferred. We use the concept of Heat capacity to do this. Heat capacity of a substance describes the amount of heat that can be absorbed by the substance for a given unit rise in temperature (unit = 1 K). It can be expressed in general as : Heat capacity of a substance describes the amount of heat that can be absorbed by the substance for a given unit rise in temperature (unit = 1 K). It can be expressed in general as: The Heat capacity can be expressed as a function of the amount of material. The heat capacity per mole is called the molar heat capacity (units J K1mol1). The heat capacity per gram is called the specific heat capacity (or just specific heat) (units J K1g1). It can simply be the capacity of the unit object. For example, a calorimeter (made up of or containing several materials, container, insulation, water, etc.) will have a heat capacity unique to it. It is not completely correct to talk of heat capacity in terms of q since q is not a state function. We are better off using the state functions H rather than q. We assume that no electrical or mechanical work w' is being done. At constant pressure, we measure heat as H = qp, and hence the heat capacity we need to use is Cp, where we define Thus, we can write (at constant P) H = CpT The units of Cp are J/K or J/C (remember that T is the same whether its measured in C or in K). Sometimes, we tabulate heat capacities in per mole or per gram values. If this is the case, we need to multiply the molar heat capacity by the number of moles or the gram heat capacity (specific heat) by the number of grams to get the total heat capacity. In these cases, we can think of modifying our equation for enthalpy to be H = nCpT (for molar heat capacity values) OR H = mCpT .(for specific heat values) Obviously, these equations hold Cp to be independent of temperature. In reality, that's a reasonable estimate for our purposes but is not strictly correct. H = 52.2 kJ/mol (ENDOTHERMIC)
Thermochemistry (calorimetry) Now, Let's quickly review a few definitions we will need to use in this section. Calorimetry Study of heat absorbed or evolved in chemical reactions Calorimeter Device used in Calorimetry to measure heat processes (normally thermally insulated from the surroundings) Heat Capacity Amount of heat to raise an object or a given amount of substance by 1C (1K). Molar Heat Capacity Amount of heat to raise one mole of a substance by 1C (1K). (water has molar heat capacity of 75.4 J K 1mol 1 .
Specific Heat Amount of heat required to raise one gram by 1C (1K). (water has specific heat of 1 cal K 1g 1 or 4.18 J K 1g 1
Let's try an example: A sample of 50. mL of a 0.20 M solution of HCl was mixed with 50. mL of 0.20 M NaOH in a coffee cup calorimeter. The initial temperature of both solutions was 22.2C. After mixing, the temperature rose to 23.5C. What is the enthalpy change for the neutralization reaction which occurred? To Start, we need to define the system. Since this case involves a reaction in a liquid solution where the chemicals are intimately involved with the solvent (water), we cannot really separate the water from the chemicals. We use the chemicals and water as the system. The insulated cup is the boundary between the system and the surroundings and we will assume that the cup itself absorbs no heat and that no heat passes through the cup to the surroundings. We have thus, a two step process to deal with. The reaction occurs, warming the system. Since no heat was evolved to the surroundings (coffee cup insulation prevented it) the value of q and of H are zero since w is negligibly small (no volume change in a liquid). For that matter, U is zero too (See Internal Energy at the end of this section). The system must be returned to the starting temperature by extracting heat from it or adding heat to it. Thus, the T we calculate is of the step where the system is returned to its original state. H3O+(aq) + OH(aq) 2 H2O(l)
Total volume = 100. mL, dilute aqueous so we assume density = 1.00 g/mL mass of solution: msol = 100. mL 1.00 g/mL = 100. g For the cooling process: T = Tf Ti = 22.2C 23.5C = 1.3C (= 1.3 K) The temperature was lowered from it's high value back down to its starting point. Thus, we expect a negative temperature change. Another way of viewing this: We removed heat from the system to return it to its starting point. Hence, q and H are negative (the reaction is exothermic). This temperature change is happening to the system. qp = CpT = mCm T (Cm is specific heat, i.e., heat capacity per gram) NOTE: to figure out which equation you need simply look at the units of the quantities you have and figure out how to cancel out the undesired units by the correct combination of C with the values you have. In this case, we have qp = 4.18 J K-1g-1 100. g -1.3 K = -540. J # mol H3O+ = # mol HCl = 0.50 L 0.20 M = 0.010 mol. H' = qp = -540 J
Note that the H' shown here is for the whole reaction amount. It is not a molar amount; hence, the prime on the H'. I will use the prime to distinguish the 'extrinsic' quantity and no prime to show an 'intrinsic' quantity (molar). -540 J H = ---------- = -54. kJ/mol H3O+ 0.010 mol equation has 1 mol H3O+ 1 mol reaction or it might also look like: H3O+(aq) + OH-(aq) ---> H2O(l) H = -27. kJ/mol reaction so we can write H = -54. kJ/mol reaction.
Our test calorimeter is not that accurate (significant heat loss to the surroundings). The true H for the first reaction is -56.02 kJ/mol and is valid for the heat of neutralization of any strong acid with a
strong base in water.
Here's another example: 0.510 g of ethanol is burned in a flame calorimeter containing 1200 g of water. The water is initially at 22.46C and is warmed up to 25.52C as a result of the reaction. What is the H for one mole of ethanol? In this case, we can more easily separate the gas reaction mixture from the water in the calorimeter which absorbs (all) the heat from the reaction. Therefore, we can use the chemicals themselves as the system. In this case, the insulation in the calorimeter serves to isolate a small portion of the surroundings (the water) so we can measure its temperature change. C2H5OH(l) + 3 O2(g) ---> 2CO2(g) + 3 H2O(l) Twater = 25.52C - 22.46C = 3.06C = 3.06 K we have grams of water so use a C which has grams to cancel out. This is the total heat absorbed by the water and is the negative of the heat evolved by the system. Hence, H' = qp = -15.3 kJ 0.510 g ethanol 46.05 g/mol H = qp = -15.3 kJ 0.0111 mol ethanol (1 mol ethanol in 1 mole reaction) qp = -1380 kJ/mol ethanol or simply -1380 kJ/mol. Note this reaction is written such that one mole of a reactant is burned completely in oxygen. This type of reaction is called a combustion reaction and the H is called a heat of combustion. We could simply tabulate Hcomb and know that the reaction is as written above. Standard Enthalpy Changes Reaction energies depend on the conditions under which they were measured. In order to be able to record energies and tabulate them in a way that others can make sense of them, we define a standard state. This standard state is then used to define the state of both the reactants (before reaction) and products (after reaction). Recall that since H and U do not depend on the path, only the initial and final state. Hence this is a useful thing to do. Thermodynamic Standard state should not be confused with the standard state for Ideal Gases. They aren't the same. Standard State: Pressure = 100 kPa (1 bar)* Concentration = 1 mol/L, (solutions) [Temperature = Normally, 25 C, ] We'll see later that a simpler definition for standard state is "activity = 1"Actually, Temperature is not part of the definition. It is merely the experimental temperature for which most of the results are tabulated. We indicate Thermodynamic quantities measured at standard conditions by using the superscript zero (sometimes pronounced "naught") as in H. eg. CH4(g) + 2 O2(g) ---> CO2(g) + 2 H2O(l) H = -890.4 kJ/mol = -1380 kJ/mol ethanol = 0.0111 mol ethanol
NOTE: all the reactants and products are in their standard state. Reactions may well occur at temperatures far different from Standard State but we always start with the reactants at Std State and then perform the reaction at whatever conditions desired, then return the products to their standard state, all the while, recording carefully the amount of energy used in each of the steps. The overall effect is the standard enthalpies (or internal energies) of reaction. This is valid, of course, since H is a state function. There are several ways of recording standard enthalpies of reaction. Recall that the enthalpy of a reaction depends on how it is written, (if all coefficients are doubled, so is the H of reaction. Hence,
we either need to include the complete balance chemical reaction with the tabulated values of H or define a 'standard' way of writing the equations. The latter is the way we do it. We've already seen two such reactions and their enthalpy changes. Formation reactions are written by default such that the compound of interest is formed from its constituent elements in their standard states. (see the formation of water reaction: H2(g) + O2(g) ---> H2O(l) Hf = -286. kJ/mol
We could also specify a reaction where the compound of interest is a reactant consumed completely, for example in a combustion with excess oxygen. C2H6(g) + 7/2 O2(g) ---> 2 CO2(g) + 3 H2O(l) Hcomb = -1560.4 kJ/mol
Since both of these types of reactions are now well defined, we can re-create the chemical equation exactly knowing only the compound of interest and the type of reaction. Hence, the values can be tabulated without writing the complete reaction.
Hess' Law Since enthalpy is a state function, we can go to products via several routes and the enthalpy change will still be the same. or The enthalpy change in a reaction does not depend on the reaction pathway. Let's look at the example of ethane burning in excess oxygen to produce carbon dioxide and water. We can consider the reaction to occur in one complete process [T] or in three distinct steps [1], [2] and [3]. In either case, the total enthalpy change should be the same.
[1] Ethane is heated to drive off H2. C2H6 ---> C2H4 + H2 [2] Ethene is burned in excess O2. C2H4 + 3 O2 ---> 2 CO2 + 2 H2O [3] H2 is burned in excess O2. H2 + 1/2 O2 ---> H2O [T] overall reaction is sum [1]+[2]+[3] C2H6 + 3.5 O2 ---> 2 CO2 + 3 H2O H3 = -285.8 kJ H2 = -1410.8 kJ H1 = +136.2 kJ
____________________ HT = -1560.4 kJ
We can use this idea to calculate enthalpies of reactions for reactions where measurement is impractical. Example: Use the following standard enthalpies of combustion to calculate the enthalpy change for the following reaction C(s) + 2 H2(g)---> CH4(g) 1) Hcomb(C) 2) Hcomb(H2) = -393.5 kJ = -285.8 kJ H = ?
3) Hcomb(CH4) = -890.4 kJ Knowing the standard format for the combustion reaction (1 mol of reactant burns to completion in oxygen to produce carbon dioxide and water) we can easily write down the three reactions. 1) 2) 3) C(s) + O2(g) ---> CO2(g) H2(g) + O2(g) ---> H2O(g) CH4(g) + 2 O2(g) ---> CO2(g) + 2H2O(l) H = -393.5 kJ/mol H = -285.8 kJ/mol H = -890.4 kJ/mol
Now lets add up the three reactions to give the desired reaction
1)
C(s) + O2(g) ---> CO2(g)
H = -393.5 kJ/mol H = -285.8 kJ/mol] H = +890.4 kJ/mol
2)2[H2(g) + O2(g) ---> H2O(g) 3) CO2(g) + 2H2O(l) ---> CH4(g) + 2 O2(g)
________________________________________________________________ C(s) + 2 H2(g) ---> CH4(g) H = -74.7 kJ/mol
Standard Enthalpies of Formation Enthalpy change for the reaction in which one mole of a compound is formed from its elements in their standard states. Ex. C(graphite) + O2(g) ---> CO2 H2(g) + O2 ---> H2O(l) C(graphite) + 2H2(g) ---> CH4(g) Hf = -393.5 kJ/mol Hf = -285.8 kJ/mol Hf = -74.7 kJ/mol
Question: What will be the standard enthalpy of formation of an element in its standard state [like O2(g)]? Answer: zero. Now let's use these with Hess' law to determine the reaction enthalpy for the following CH4(g) + 2 O2(g) ---> CO2(g) + 2H2O(l) (combustion of methane) We could chose to break down the reactants to their constituent elements in their standard state and then reconstruct them into the products. CH4(g) + 2 O2(g) ---> C(s) + 2H2(g) + 2O2(g) C(s) + 2H2(g) + 2O2(g) ---> CO2(g) + 2H2O(l) From Hess' law, we have, Notice that the following is true: H2 = - sum of Std enthalpies of formation of reactants H3 = + sum of Std enthalpies of formation of products So now, we can write: For the combustion of methane we're dealing with here, we can write H1 = [Hf(CO2) + 2 Hf(H2O)] [Hf(CH4) + 2 Hf(O2)] We can look up the values for Heat of formation in any standard reference book. H1 = [(393.5 kJ) + 2 (285.8 kJ)] [(74.7kJ) + 2 (0)] H1 = 890.4 kJ In general, we can write: H = Hf(prod) Hf(reactants) A more completely mathematically correct equation is written as follows H1 = H2 + H3 H2 H3 H1
This (these) reaction is merely a special application of Hess' Law whereby we are using Heats of formation as the heats to add up.
What are elements in their standard states? How do we decide which ones to use? Take hydrogen: Most hydrogen exists at standard conditions as H2(g) [minute amounts may exist as H(g)] so we choose the most common form at normal (standard) conditions. Now try Carbon: Obviously, carbon is solid at Std Conditions but do we choose graphite, diamond, bucky balls (Buckminster Fullerenes)? Graphite is the defined standard, it is more common and more stable than diamond. Let's try a few examples to get some practice with these calculations: What is the standard enthalpy change for 4 NH3(g) + 5 O2(g) ---> 4NO(g) + 6 H2O(l) ?
H = Hf(prod) - Hf(reactants) H = [4 H(NO) + 6 H(H2O)] - [4 H(NH3) + 5 H(O2)] H = [4 (90.25 kJ/mol) + 6 (-285.83 kJ/mol)] - [4 (-46.11 kJ/mol) + 5 (0)] H = -1169.54 kJ/mol Now here's another one. B5H9(g) reacts exothermically with O2 to form B2O3(s) and water. What is the standard enthalpy change for the reaction of 1 mol of B5H9(g)? balance this B5H9(g) + O2(g) ---> B2O3(s) + H2O(l)
2 B5H9(g) + 12 O2(g) ---> 5 B2O3(s) + 9 H2O(l) for 1mol 1 B5H9(g) + 6 O2(g) ---> 5/2 B2O3(s) + 9/2 H2O(l)
H = Hf(prod) - Hf(reactants) H = [5/2 H(B2O3) + 9/2 H(H2O)] - [H(B5H9) + 6 H(O2)] H = [5/2 (-1272.77) + 9/2 (-285.83)] - [(73.2) + 6 (0)] H = -4541.4 kJ/mol
1 mol of benzene burns in air at standard state conditions and gives off 3267 kJ of heat. What is the enthalpy of formation of C6H6? C6H6(l) + 7.5O2(g) ---> 6 CO2(g) + 3 H2O(l) Hcomb = -3267 kJ/mol Hcomb = Hf(prod) - Hf(reactants) + 3 Hf(H2O,l)] -
-3267 kJ/mol = [6 Hf(CO2,g)
[Hf(C6H6,l) + 7.5 Hf(O2,g)] Substitute in values from data table. -3267 kJ/mol = [6 (-393.5) + 3 (-285.83)] -
[Hf(C6H6,l) + 7.5 (0)] Solve for the only unknown: Hf(C6H6,l) = 49 kJ/mol One could look at the Hf for a compound and tell if there is much chemical energy stored in it or not. If the heat of formation is positive then energy was stored (less stable) in it's production, if negative, it lost energy (more stable) when produced. Temperature change and Enthalpy
The standard enthalpy data we find tabulated in thermodynamic tables is all for data at 25C. This data may not be exactly the information we need. What if a reaction doesn't actually happen at standard 25C. We have two options: 1, we can assume that there is no significant difference in enthalpy change or, 2, we can calculate the effect. In cases where there is only small temperature changes the former option may not be too bad. However, in some cases, especially where the temperature of reaction is far from 25C we should take the latter option. Consider a reaction between chemicals A and B to produce C and D at some temperature T. We could calculate the H for the process 2 using standard thermodynamic data. Steps 1 and 2 involve temperature changes and we can calculate the H for these steps if we know the heat capacities of the compounds involved. According to Hess' law, the overall enthalpy change for the reaction at temperature T is the sum of the steps 1, 2 and 3. Step 1: This is simply a temperature change and we can calculate the enthalpy change using the heat capacities of A and B. H1 = {aCP(A) + bCP(B)}T1 (298 T)
Step 2: H2 = H298 (calculate from whatever means possible, for example you could use Hf values.) Step 3: Like step 1, this is simply a temperature change. Calculate the enthalpy change using the heat capacities of C and D. Note that the process is the reverse direction of step 1 and the sign of the temperature change is opposite. H3 = {cCP(C) + dCP(D)}T3 Step T: HT = H1 + H298 + H3 = {aCP(A) + bCP(B)}T1 + H298 + {cCP(C) + dCP(D)}T3 = {cCP(C) + dCP(D) [aCP(A) + bCP(B)]}T3 + H298 = {CP}T3 + H298 The change in heat capacity (capacity of products capacity of reactants) is a actually slightly dependent on temperature and hence this formulation is not valid if the temperature T is very different from standard temp. (298 K). In most cases, the temperature effect is not extremely large but that is not general. Like the general concepts involved in Hess' Law, we simply add up the heats of the processes involved in order to determine the overall heat. This is merely another special application of Hess' Law. Internal Energy Internal energy of the system is represented by the symbol U. It is a more general description of the energy of the system than enthalpy. We can never measure the absolute energy of a system but we can measure changes in the internal energy of the system using the state function U = Ufinal - Uinitial. Since we can never actually measure either Uinitial nor Ufinal we measure, instead, the energy transferred into or out of the system in the form of heat and work to obtain. U = q + w ( = heat transferred + work done) This is, in fact, the mathematical expression of the First Law of Thermodynamics and is completely general, not relying on any of the restrictions we placed on enthalpy calculations. It turns out that while neither q nor w are state functions, their sum is. Example: If 15 kJ of work is done by the surroundings on the system and the system looses 10 kJ heat to the surroundings then U = -10 kJ + 15 kJ = +5 kJ Chemical reactions often involve changes in Volume (exp. gases) (T 298) NOTE: T1= T3
C3H8(g) + 5 O2(g) ---> 3 CO2(g) + 4 H2O(g) 6 mol gas 7 mol gas ====> increase in volume at const. P If this reaction is to happen, it needs to push back the rest of universe (atmosphere) to make room for itself. That's a force( pressure * area) * displacement = work. work done => pV (since the system did the work, it's negative) w = - PsurrV (gas expanding against an external pressure Psurr.)
U = q + w = q - PsurrV or qp = U + PsurrV in this case, qp = U + ngRT (the only volume increase came from the fact that the number of moles of gas is different in product than in reactants).
In a closed container (no volume change) we have V = 0 U = qv. subscript v refers to constant volume conditions. Enthalpy is now better defined as H = U + PV Changes in enthalpy are H = U + PV since we normally work in a fixed pressure (atmosphere) situation, we can rewrite this as H = U + PV or we can write: so:
To do these measurements under general conditions, we need to expand a few previous definitions. First, lets look at heat capacity. At constant pressure, we measure heat as H = qp, and hence the heat capacity we need to use is Cp, where we define At constant volume, the heat change we measure is U = qv and the heat capacity CV is defined as In many substances, the two heat capacities differ considerably. Consider ideal gases. We have H U = (PV) = RT. Therefore CP CV = R. Solids and liquids often have values of CP and CV that don't differ much. On the other hand there is no consistent rule. Take for example water and benzene; for water, CP CV = 0.075R, while for benzene we have CP CV = 5.1R. let's do an example where we look at the PV work done. When 2.00 mol of SO2(g) react completely with 1.00 mol O2(g) to form 2.00 mol of SO3(g) at 25C and constant pressure of 1.00 atm, 198 kJ of energy is released as heat. Calculate U and H for this reaction. 2 SO2(g) + 1 O2(g) ---> 2 SO3(g) The heat released is |qp| since it was measured under constant pressure conditions. so we can quickly say H' = 198 kJ (negative sign since the heat is released, i.e., exothermic)
H = 198 kJ/mol (trivial in this case since the molar quantities given exactly match the numbers in the equation) U' = qp + w = qp PV (total PV work) or (since we have the molar values) U = H - PV (remember, this is the PV work per mole which just happens to equal the total PV work in this case) Since we have constant P and T conditions, the only change in PV will be due to change in numbers of moles of gas molecules. We can use the stoichiometry from the equation as written to determine PV : U = H - nRT or H = U + nRT
Note: Either of these two equations above are valid for any situation where the T and P are constant but where the number of moles of gas molecules changes because of stoichiometry. Pick one and learn it. n = #moles prod gas #moles reactant gas = 2 3 = 1 mol. U = -198 kJ/mol + -(-1 * 8.31451 J K-1mol-1 * 298 K) = -198 kJ/mol + 2.48 kJ/mol = -196 kJ/mol
Thermochemistry Enthalpy ( H) is the amount of heat content.
Heat content is accounted for by a change in "heat flow" or enthalpy of the reaction system. Endothermic reaction: H > 0 (i.e., H products >H reactants). Heat absorbed goes to increase the enthalpy of the reaction system. Exothermic reaction: H < 0 (i.e., H products < H reactants). Heat is evolved at the expense of the reaction system. Thermochemical Equation: specify CH4(g) + 2O2(g) H = -890 kJ 6.00kJ + H2O(s) H = +6.00kJ H in kilojoules/mole.
CO2(g) + 2H2O(l) + 890.3 kJ H2O(l) H is written as a product or reactant !
! In some textbooks
The preceding is based upon the Law of Conservation of Energy (James Joule, 1818-1889, Joule also developed the First Law of Thermodynamics): energy is neither created nor destroyed in ordinary chemical or physical changes. Quantitative H H = qreaction mixture (at constant temperature only) q = (m)( t)(Cp) q = heat absorbed by the water in joules (J) m = mass of substance t = tfinal - tinitial Cp = specific heat of water = 4.184 When using moles, molar heat capacity is used. The units are 1 cal = 4.184 J Calorimetry
Coffee-cup calorimeter (only used for reactions in solution, must be at constant pressure) qreaction=-qwater Bomb calorimeter (reaction gases, and must have constant volume) qreaction=-(qwater+qbomb) qbomb=C t, where C is the calorimeter constant (Cv of bomb x mass of bomb, really same equation) H vs. E for chemical reactions H=qp since E=qp-P V substituting gives H= E+P V where P will usually be in atmospheric pressure, and Laws of Thermochemistry The magnitude of H is directly proportional to the amount of reactant or product. -Thus H can be used as a conversion factor in a balanced equation to obtain amounts of reactant/product or H itself. (mole to mole ratio's). H for a reaction is equal in magnitude but opposite in sign to Problems 1: H Calculation H H for the reverse reaction.
V is volume change at that pressure.
When 1 mol of methane is burned at constant pressure, 890.3kJ of energy is released as heat. Calculate for a process in which a 5.8 gram sample of methane is burned at constant pressure. CH4(g) + 2O2(g) CO2(g) + 2H2O(l) + 890.3 kJ = 320 kJ H = -320 kJ For the reaction of methane with oxygen given in the notes, calculate the consumed in the process. = 81 kJ H= -81kJ
H in kJ if 5.8 grams of oxygen are
Ammonium nitrate, NH4NO3, is commonly used as an explosive. It decomposes by the following reaction: NH4NO3 N2O(g) + 2 H2O(g) + 37.0kJ
H = -37.0 kJ If 72.0 grams of H2O are formed from the reaction, how much heat was released? = 73.9 kJ Hess' Law: The value of function). Htotal = H1 + H2 H for a reaction is the same whether it occurs directly or in a series of steps (state
often used to calculate H for one step, knowing H for all steps and for the overall reaction. **All of the laws of thermochemistry follow from the fact that the enthalpy H of a substance is one of its properties.** Heats of Formation Molar heat of formation ( Hf) is equal to the enthalpy change, H when one mole of the compound is formed from the elements in their stable forms at 25oC and 1 atm is Ho (pronounced 'delta h naught'). Ho of a solution is of a 1M solution, at 1 atm and 25 oC. Heats of formation are usually negative quantities. H= Hf products Hf reactants To apply the above relation, use the following rules:
The contribution for each compound is found by multiplying the heat of formation in kJ per mole by the number of moles of compound, given by its coefficient in the balanced equation. Heats of formation can be found in appendix A-4 Any element in its stable form is omitted. Can also apply to heats of formation to ions. Arbitrarily assign H+ ion to be zero. Hf H+(aq) = 0
Having established the above, a scale can be established with Hydrogen ion as the base. Calculate H0rxn for the following reaction.
2 C3H6(g) + 9 O2(g) 6 CO2(g) + 6 H2O(l) *Appendix 4* Hrxn = Hf products Hf reactants Hrxn = [ 6 H2O(l) + 6 CO2(g) ] - [ 2 C3H6(g) + 9 O2(g) ] Hrxn = [ 6(-286 kJ) + 6 (-393.2 kJ) ] - [ 2(20.9 kJ) + 9(0)]
Hrxn = [ -1716 kJ + -2361 kJ ] - [41.8 kJ ] Hrxn = Calculate = 2059 H0 for 2Al(s) + Cr2O3(s) Al2O3(s) + 2Cr(s).
Compare this reaction to sample exercise 6.10, "thermite" reaction. Which reaction yields more energy per gram of metal formed? Hrxn = [ Al2O3(s) + 2 Cr(s) ] - [ 2 Al(s) + Cr2O3(s) ] Hrxn = [ (-1676 kJ) + 2(0 kJ) ] - [ 2(0 kJ) + -1128 kJ ] Hrxn = [ -1676 kJ ] - [ -1128 kJ ] Hrxn = = -10.1
Hrxn = = -15.75 "Thermite" reaction releases more energy per gram of metal formed.
Вам также может понравиться
- 1422 Notes Full 2010Документ365 страниц1422 Notes Full 2010Sreedevi KrishnakumarОценок пока нет
- Chem 1302 Midterm ReviewДокумент9 страницChem 1302 Midterm ReviewSaddi MahmoodОценок пока нет
- Physical Biochemistry Lecture NotesДокумент9 страницPhysical Biochemistry Lecture Noteschc300Оценок пока нет
- Astm d396 Fuel OilsДокумент5 страницAstm d396 Fuel Oilsjhon.rojas.ery1489Оценок пока нет
- Chapter 6 Lecture NotesДокумент10 страницChapter 6 Lecture NotesAhmad KamalОценок пока нет
- Chapter 17 Outline Chem 1062: Probability To States of High ProbabilityДокумент9 страницChapter 17 Outline Chem 1062: Probability To States of High Probabilityaq300Оценок пока нет
- CH 101 Class 09 Energetics 01 PDFДокумент12 страницCH 101 Class 09 Energetics 01 PDFliz_hobbs79Оценок пока нет
- Thermodynamics: Lecture 2 Review of Lecture 1Документ10 страницThermodynamics: Lecture 2 Review of Lecture 1Lexter Gomez GabicaОценок пока нет
- ThermochemistryДокумент5 страницThermochemistryjoelsantos1981Оценок пока нет
- Chemical ThermodynamicsДокумент1 страницаChemical ThermodynamicsYukiko HachiОценок пока нет
- Mole: 1 Mole of A Substance Contains Avogadro's Number (N 6.02E23)Документ53 страницыMole: 1 Mole of A Substance Contains Avogadro's Number (N 6.02E23)Juan Carlos Gonzalez LОценок пока нет
- Thermo ChemistryДокумент8 страницThermo ChemistryANGELA JOIE BAIZASОценок пока нет
- Thermochemistry or Junior IntermideateДокумент48 страницThermochemistry or Junior IntermideateEvs GoudОценок пока нет
- CO3 ThermodynamicsДокумент72 страницыCO3 ThermodynamicsRonna IturaldeОценок пока нет
- Thermochemistry NotesДокумент5 страницThermochemistry NotesNephtali Pinos-anОценок пока нет
- Met Thermo 2Документ10 страницMet Thermo 2msahisriОценок пока нет
- Chapter2 - Thermodynamics - Why Things ChangeДокумент25 страницChapter2 - Thermodynamics - Why Things ChangeDylan HamОценок пока нет
- Thermochemistry Answers RemovedДокумент11 страницThermochemistry Answers Removedapi-327309463Оценок пока нет
- UntitledДокумент16 страницUntitledapi-233404189100% (1)
- Chem 2Документ82 страницыChem 2César ArenasОценок пока нет
- 8 1 Problem SetДокумент11 страниц8 1 Problem Setapi-182809945Оценок пока нет
- Thermodynamics: Basic DefinitionsДокумент8 страницThermodynamics: Basic DefinitionsAnu RadhaОценок пока нет
- Partial Pressure Equilibrium and EntropyДокумент6 страницPartial Pressure Equilibrium and EntropyJoeОценок пока нет
- Lecture - 2 - 1st - Law of ThermodynamicsДокумент21 страницаLecture - 2 - 1st - Law of ThermodynamicsahmedОценок пока нет
- CHEM 1902 Lecture 1 RevisedДокумент9 страницCHEM 1902 Lecture 1 RevisedRamona NeeОценок пока нет
- ThermodynamicsДокумент62 страницыThermodynamicsHarini MeiyappanОценок пока нет
- Book Summary: A. The Nature of Energy and Types of EnergyДокумент9 страницBook Summary: A. The Nature of Energy and Types of EnergyFildzahОценок пока нет
- Ch06 SlidesДокумент73 страницыCh06 SlidesbeelzeburtonОценок пока нет
- Chapter 19 Chemical ThermodynamicsДокумент8 страницChapter 19 Chemical ThermodynamicsRSLОценок пока нет
- Thermodynamics First LawДокумент43 страницыThermodynamics First LawMehenaz JahanОценок пока нет
- Energy - CHEM 106Документ29 страницEnergy - CHEM 106Eunice MaeОценок пока нет
- Study ChemДокумент13 страницStudy ChemJanthina Rose AusteroОценок пока нет
- Chapter 6 ThermochemistryДокумент6 страницChapter 6 ThermochemistryKevin HuangОценок пока нет
- Me 1007Документ23 страницыMe 1007Bhuvanesh M.PОценок пока нет
- Me 291 Introduction To Mechechanical EngineeringДокумент11 страницMe 291 Introduction To Mechechanical EngineeringDaniel Deng KuolОценок пока нет
- Eq o o o o Eq: ND RDДокумент8 страницEq o o o o Eq: ND RDEl-Sayed MohammedОценок пока нет
- CH 8 - Energy, Enthalpy, and ThermochemistryДокумент64 страницыCH 8 - Energy, Enthalpy, and ThermochemistryCharbel RahmeОценок пока нет
- Physical Chemistry (Part-2)Документ73 страницыPhysical Chemistry (Part-2)RSLОценок пока нет
- Which Area of Chemistry Is Concerned With The Topic?: THERMOCHEMISTRY: Energy Changes in Chemical ReactionsДокумент19 страницWhich Area of Chemistry Is Concerned With The Topic?: THERMOCHEMISTRY: Energy Changes in Chemical ReactionsSahada KanapiyaОценок пока нет
- Chemistry 223: Work, Heat, and Energy: 1. Zeroth Law of ThermodynamicsДокумент10 страницChemistry 223: Work, Heat, and Energy: 1. Zeroth Law of ThermodynamicsLakshyaОценок пока нет
- Genchem NotesДокумент8 страницGenchem NotesKarl BayaninОценок пока нет
- QuestionsДокумент8 страницQuestionsAntonioОценок пока нет
- The First Law and Other Basic Concepts (Part 1)Документ23 страницыThe First Law and Other Basic Concepts (Part 1)Ragil BudiartoОценок пока нет
- XI CH 5 NotesДокумент4 страницыXI CH 5 Notesiroonmaan123Оценок пока нет
- ScriptДокумент10 страницScriptNamrata SharmaОценок пока нет
- Unit 4: Thermochemistry and Nuclear Chemistry: Initial FinalДокумент21 страницаUnit 4: Thermochemistry and Nuclear Chemistry: Initial FinalPankaj KumarОценок пока нет
- Hukum Pertama TermodinamikaДокумент123 страницыHukum Pertama TermodinamikaLia TrisnawatiОценок пока нет
- DP ThermodynamicsДокумент24 страницыDP ThermodynamicsYash AkhauriОценок пока нет
- EntropyДокумент35 страницEntropyRavi PaswanОценок пока нет
- ThermodynamicsДокумент2 страницыThermodynamicsPatrick Lorenze M. ReyesОценок пока нет
- Definition of ThermodynamicsДокумент8 страницDefinition of ThermodynamicsLis MiaОценок пока нет
- 1ST Law of ThermodynamicsДокумент7 страниц1ST Law of ThermodynamicsKen BorjaОценок пока нет
- The Key: Thermochemistry Is The Branch of Physical Chemistry Which Deals With The Thermal or Heat ChangesДокумент23 страницыThe Key: Thermochemistry Is The Branch of Physical Chemistry Which Deals With The Thermal or Heat ChangesSachin KumarОценок пока нет
- BasicsДокумент65 страницBasicsBas RamuОценок пока нет
- The First Law of Thermodynamics - 230727 - 113816Документ41 страницаThe First Law of Thermodynamics - 230727 - 113816Tshiamo MotaungОценок пока нет
- Chapter 6 IM Chang 11eДокумент8 страницChapter 6 IM Chang 11eSelma MeloОценок пока нет
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4От Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4Оценок пока нет
- Pressure, Heat and Temperature - Physics for Kids - 5th Grade | Children's Physics BooksОт EverandPressure, Heat and Temperature - Physics for Kids - 5th Grade | Children's Physics BooksОценок пока нет
- Loss of Appetite in ChildrenДокумент13 страницLoss of Appetite in ChildrenDrazelle PangilinanОценок пока нет
- Energy Matter BondingДокумент27 страницEnergy Matter BondingDrazelle PangilinanОценок пока нет
- MolalityДокумент3 страницыMolalityDrazelle PangilinanОценок пока нет
- Energy Matter BondingДокумент27 страницEnergy Matter BondingDrazelle PangilinanОценок пока нет
- ThermochemistryДокумент13 страницThermochemistryDrazelle PangilinanОценок пока нет
- End of LifeДокумент75 страницEnd of LifeabduolОценок пока нет
- End of LifeДокумент75 страницEnd of LifeabduolОценок пока нет
- Basic StoichiometryДокумент3 страницыBasic StoichiometryDrazelle PangilinanОценок пока нет
- Cable Design ParametersДокумент6 страницCable Design ParametersDan HawkОценок пока нет
- d01 PORTAL SPLAPP PDF Useful Informations ScrutinyCases 2013 2014 Faridabad (W) R PDFДокумент43 страницыd01 PORTAL SPLAPP PDF Useful Informations ScrutinyCases 2013 2014 Faridabad (W) R PDFAnjali Srivastava100% (1)
- WO2008033224A1 Polycaprolactone PolyurethaneДокумент31 страницаWO2008033224A1 Polycaprolactone PolyurethaneGİZEM DEMİRОценок пока нет
- Chapt 4 Phase EquilibriumДокумент47 страницChapt 4 Phase EquilibriumNikunj Yagnik100% (1)
- Experiments 3,4,5Документ13 страницExperiments 3,4,5Athirah JamalludinОценок пока нет
- Shake and Bake One Pot MethamphetamineexperimentДокумент4 страницыShake and Bake One Pot MethamphetamineexperimentCep Oboz Cc'settand Nalaktack0% (1)
- Specific Heat of Liquids and FluidsДокумент4 страницыSpecific Heat of Liquids and FluidsnicoОценок пока нет
- TH-005-Examples of Chapter Five PDFДокумент7 страницTH-005-Examples of Chapter Five PDFLinda LCОценок пока нет
- Deep DrawnДокумент66 страницDeep DrawnMukesh KumarОценок пока нет
- 11 Repair KitsДокумент2 страницы11 Repair KitsbuddhansamratОценок пока нет
- The Design, Analysis and Construction of Tensile Fabric StructuresДокумент26 страницThe Design, Analysis and Construction of Tensile Fabric Structurespradeep vermaОценок пока нет
- Instrumental Presentation, Esma, 27.8.16Документ14 страницInstrumental Presentation, Esma, 27.8.16John Paul DenajibaОценок пока нет
- The Iodimetric Method of Determining Lactose in MilkДокумент4 страницыThe Iodimetric Method of Determining Lactose in MilkgustavoesanchezОценок пока нет
- Additives Used in The Production of PET: Antimony Tri-Acetate, CatalystДокумент4 страницыAdditives Used in The Production of PET: Antimony Tri-Acetate, CatalystHAmza RiAzОценок пока нет
- Chapter 5 - GasesДокумент72 страницыChapter 5 - GasesAmbar WatiОценок пока нет
- A Report On Structural Analysis and Design On Residental BuildingДокумент82 страницыA Report On Structural Analysis and Design On Residental BuildingCharchitОценок пока нет
- Novel Hybrid Structural Core Sandwich Materials For Aircraft ApplicationsДокумент4 страницыNovel Hybrid Structural Core Sandwich Materials For Aircraft Applicationsphd.meethaqОценок пока нет
- Insulation R ValuesДокумент2 страницыInsulation R Valueshiren13183Оценок пока нет
- Amorphous MaterialsДокумент12 страницAmorphous MaterialsdevendrakphyОценок пока нет
- Performance Based Design For Fire SafetyДокумент23 страницыPerformance Based Design For Fire SafetyTharanga Pradeep100% (1)
- Heat Calculations Practice 2 PDFДокумент2 страницыHeat Calculations Practice 2 PDFRizqi HidayatОценок пока нет
- Mechanical Properties of Heat Affected ZoneДокумент9 страницMechanical Properties of Heat Affected ZoneLeandro VaccariОценок пока нет
- CarbonCure Technical Note - Types of Concrete CarbonationДокумент4 страницыCarbonCure Technical Note - Types of Concrete CarbonationSakineОценок пока нет
- 2020Документ7 страниц2020JEORJEОценок пока нет
- Industrial Waste Management: Che 3101 G1 1/N 00 1/1Документ2 страницыIndustrial Waste Management: Che 3101 G1 1/N 00 1/1JAN JERICHO MENTOYОценок пока нет
- Chemistry Paper 3 SLДокумент32 страницыChemistry Paper 3 SLNate ChenОценок пока нет
- TDS STEELGRID HR SYSTEM - Rev 08 - Jun 2014Документ2 страницыTDS STEELGRID HR SYSTEM - Rev 08 - Jun 2014mandeep571Оценок пока нет
- Cicli Corsa - Racing Bikes: Listino Prezzi - Price ListДокумент6 страницCicli Corsa - Racing Bikes: Listino Prezzi - Price ListcarbonabikesОценок пока нет