Академический Документы
Профессиональный Документы
Культура Документы
04 Conformational Anal 1
Загружено:
bidomasterИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
04 Conformational Anal 1
Загружено:
bidomasterАвторское право:
Доступные форматы
D. A.
Evans
Acyclic Conformational Analysis-1
Chem 206
http://www.courses.fas.harvard.edu/colgsas/1063
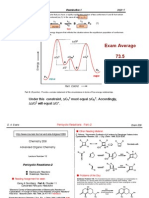
Problem 61. The following stereoselective hydroboration has been reported by Kishi in his synthesis of monensin (JACS 1979, 101, 259). Provide the stereostructure of the major product and rationalize the stereochemical outcome as indicated in the directions.
Chemistry 206 Advanced Organic Chemistry
Lecture Number 4
BH3, THF O Me Me OCH2Ph H2O2, -OH
The product ? Stereoselection: 8/1
Conformational Analysis-1
! !
Problem 68. The following stereoselective enolate alkylation has been reported by Kim (Tetrahedron Lett. 1986, 27, 943). Provide the stereostructure of the major product and rationalize the stereochemical outcome as indicated in the directions.
Me
Ethane, Propane, Butane & Pentane Conformations Simple Alkene Conformations
TsO CO2Me C4H9
LiNR2
The product ? Stereoselection: >40:1
! Reading Assignment for week A. Carey & Sundberg: Part A; Chapters 2 & 3
R. W. Hoffmann, Angew. Chem. Int. Ed. Engl. 2000, 39, 2054-2070 Conformation Design of Open-Chain Compounds (handout)
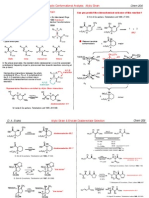
Problem 722. Carbonium ion A has been calculated to be 38 kcal/mol more stable than carbonium ion B (Jorgensen JACS 1985, 107, 1496). The profound stabilization of carbonium ions by silicon in this fashion is referred to as the "beta-silicon effect". For example, the SN1 solvolysis reaction of 1 is 10+12 times as fast as the corresponding reaction of 2. The solvolysis of 2 leads to the olefin. For a good review see: Lambert Acc. Chem. Res. 1999, 32, 183-190
R3Si vs R3 C
CH2 A
CH2
CH2 B
CH2
The Ethane Barrier Problem
F. Weinhold, Nature 2001, 411, 539-541 "A New Twist on Molecular Shape" (handout) F. M. Bickemhaupt & E. J. Baerends, Angew. Chem. Int. Ed. 2003, 42, 41834188,"The Case for Steric Repulsion Causing the Staggered Conformation in Ethane" (handout) F. Weinhold,, Angew. Chem. Int. Ed. 2003, 42, 4188-4194,"Rebuttal of the BikelhauptBaerends Case for Steric Repulsion Causing the staggered Connformation of Ethane" (handout)
Me3C H 1 SiMe3 H H
Solvolysis (CF3CH2OH)
Me k1 k2 Me3C H 2 H H
OCOCF3
= 2.4 x 10+12
OCOCF3
Part A: Identify the HOMO LUMO interactions in the SN1 reactions of 1 and 2.
1-LUMO 1-HOMO
2-LUMO 2-HOMO
D. A. Evans
Monday, September 25, 2006
D. A. Evans
Acyclic Conformational Analysis-1
Chem 206
Ethane Rotational Barrier: The FMO View
F. Weinhold, Angew. nature 2001, 411, 539-541"A New Twist on Molecular Shape"
One explanation for the rotational barrier in ethane is that better overlap is possible in the staggered conformation than in the eclipsed conformation as shown below.
In the staggered conformation there are 3 anti-periplanar CH Bonds
H C H C
! CH HOMO
H
C C
!* CH LUMO
!" CH
! CH
In the eclipsed conformation there are 3 syn-periplanar CH Bonds H C H C
! CH HOMO !" CH
H
C
H
C
!* CH LUMO ! CH
Following this argument one might conclude that:
For purposes of analysis, each eclipsed conformer may be broken up into its component destabilizing interactions. Incremental Contributions to the Barrier. Structure ethane propane Eclipsed atoms " E (kcal mol 3 (H!H) 2 (H!H) 1 (H!Me)
-1)
! The staggered conformer has a better orbital match between bonding and antibonding states. ! The staggered conformer can form more delocalized molecular orbitals. J. P. Lowe was the first to propose this explanation
"A Simple Molecuar Orbital Explanation for the Barrier to Internal Rotation in Ethane and Other Molecules" J. P. Lowe, JACS 1970, 92, 3799
+1.0 kcal mol -1 +1.0 kcal mol -1 +1.4 kcal mol -1
Me Me Me
Estimate the rotational barrier about the C1-C2 bond in isobutane
D. A. Evans
Acyclic Conformational Analysis: Butane
Chem 206
Butane
Relationship between !G and Keq and pKa
Recall that:
! G = RT Ln K
or
Using the eclipsing interactions extracted from propane & ethane we should be able to estimate all but one of the eclipsed butane conformations
H H Me Me C H H H H Me H
! G = 2.3RT Log10K
staggered conformation
C H Me
eclipsed conformation
!E=?
At 298 K:
2.3RT = 1.4 (!G in kcal Mol1 ) ! G298 = 1.4 Log10Keq
Eclipsed atoms 1 (H"H) 2 (H"Me)
! E (kcal mol -1) +1.0 kcal mol -1 +2.8 kcal mol -1
Since
pKeq = Log10Keq
# E est = 3.8 kcal mol -1 The estimated value of +3.8 agrees quite well with the value of +3.6 reported by Allinger (J. Comp. Chem. 1980, 1, 181-184)
! G298 = 1.4 pKeq
Hence, pK is proportional to the free energy change
n-Butane Torsional Energy Profile
H H HH C MeMe H C H
Keq 1.0 10 100
pKeq 0 1 2
!G 0 1.4 2.8 kcal /mol
E1 H H Me H C H Me H H Me A
Ref = 0
E2
energy
H H
Me C
+3.6
Me Me G
+5.1 Barrier?
+0.88
D. A. Evans
Acyclic Conformational Analysis: Butane
Chem 206
H H Me Me C H H H H H Me C H H H Me Me Me C H H
Butane continued
From the torsional energy profile established by Allinger, we should be able to extract the contribution of the Me"Me eclipsing interaction to the barrier:
H H Me H H H Me Me C H H
Nomenclature for staggered conformers:
staggered C conformation Me
H
eclipsed conformation
trans or t or (anti) Conformer population at 298 K: 70%
gauche(+) or g+ 15%
gauche(-) or g15%
! E = +5.1 kcal mol-1
Let's extract out the magnitide of the MeMe interaction
(Klyne, Prelog, Experientia 1960, 16, 521.)
RR C 0 R R C -60
2 (H!H) + 1 (Me!Me) = +5.1 1 (Me!Me) = +5.1 2 (H!H) 1 (Me!Me) = +3.1
Incremental Contributions to the Barrier. Eclipsed atoms 2 (H!H) 1 (Me!Me) " E (kcal mol +2.0 +3.1
R
-1)
R C
sp sc sc
+60
ac
R C 180 R -120
ac
+120 R C R
ap
Eclipsed Butane conformation
From the energy profiles of ethane, propane, and n-butane, one may extract the useful eclipsing interactions summarized below: Hierarchy of Eclipsing Interactions
Torsion angle
C R
Designation syn periplanar + syn-clinal + anti-clinal antiperiplanar - anti-clinal - syn-clinal Symbol sp + sc (g+) + ac ap (anti or t) - ac - sc (g-)
X
X Y
Y H
! E kcal mol +1.0 +1.4 +3.1
-1
Energy Maxima Energy Minima
0 30 +60 30 +120 30 180 30 -120 30 -60 30
n-Butane Conformer E2
G E1 A E1 G
H
H
H H
C H
H Me Me Me
D. A. Evans
Acyclic Conformational Analysis: Pentane
Chem 206
n-Pentane
Rotation about both the C2-C3 and C3-C4 bonds in either direction (+ or -):
Me Me H Me H
g+g+ g+t
The double-gauche pentane conformation
The new high-energy conformation: (g+g)
Me H Me Me
tg-
H Me
g+g-
Me H
H Me
Me H
Me Me H
t,t
Me Me Me H H
H
g-g-
Estimate of 1,3-Dimethyl Eclipsing Interaction
X Y
Me
tg+
Me H
Me
g-t
g-g+
G = +5.5 kcal mol -1
G = X + 2Y where: X = 1,3(MeMe) & Y = 1,3(MeH) 1,3(MeH) = Skew-butane = 0.88 kcal mol-1 1,3(Me-Me) = G 2Y = 5.5 1.76 = + 3.7 kcal mol-1
1,3(Me!Me) = + 3.7 kcal mol -1
Estimates of In-Plane 1,2 &1,3-Dimethyl Eclipsing Interactions
Me Me Me Me Me Me Me Me
3.1
~ 3.7
~3.9
~ 7.6
It may be concluded that in-plane 1,3(Me!Me) interactions are Ca +4 kcal/mol while 1,2(Me!Me) interactions are destabliizing by Ca 3 kcal/mol.
D. A. Evans
Acyclic Conformational Analysis: Natural Products
Chem 206
The syn-Pentane Interaction - Consequences
R. W. Hoffmann, Angew. Chem. Int. Ed. Engl. 2000, 39, 2054-2070 Conformation Design of Open-Chain Compounds (handout)
Lactol & Ketol Polyether Antibioitics
O Me O OH H Me Me OH O H O O Me OH Et Et OH Me
R Me R Me Me Me
R'
R'
or
Me
g-g-
Me
HO R
Me H H Me
tt
H R R' H Me
or
Me
Me
Et
R'
R !
tg
Me
R'
Ferensimycin B, R = Me Lysocellin, R = H The conformation of these structures are strongly influenced by the acyclic stereocenters and internal H-bonding
Me H H R'
H H R H
gt
Consequences for the preferred conformation of polyketide natural products
Analyze the conformation found in the crystal state of a bourgeanic acid derivative!
Alborixin R = Me; X-206 R = H Internal H-Bonding
Me Me R O C OH Me OH H Me O OH O OH OH O Me Me Me H O Me Me OH O Et Me OH
OH Me Me Me Me
O OR
O
Bourgeanic acid
Metal ion ligation sites (M = Ag, K)
Me Me O C O O R Me OH H Me O OH O OH OH O Me Me Me H O Me Me OH O Et Me OH
D. A. Evans
Conformational Analysis: Ionophore X-206/X-rays
Chem 206
X-ray of Ionophore X-206 ! H2O
Internal H-Bonding
Me Me O C O OH Me OH H Me O OH O OH OH O Me Me Me H O Me Me OH O Et Me OH
X-ray of Ionophore X-206 - Ag+ - Complex
Metal ion ligation sites (M = Ag, K)
Me Me O C O O R Me OH H Me O OH O OH OH O Me Me Me H O Me Me OH O Et Me OH
"The Total Synthesis of the Polyether Antibiotic X-206". Evans, D. A.; Bender, S. L.; Morris, J. J. Am. Chem. Soc. 1988, 110, 2506-2526.
D. A. Evans
The Gauche Effect
Chem 206
Alabugin & Zeidan, JACS 2002, 124, 3175 (pdf)
The 1,2-Dihaloethanes
X
H C H H H H H C H H
X X
X = Cl; !H = + 0.91.3 kcal/mol X = Br; !H = + 1.41.8 kcal/mol X = F; !H = 0.6-0.9 kcal/mol
Observation: While the anti conformers are favored for X = Cl, Br, the gauche conformation is prefered for 1,2-difluroethane. Explain. Relevant Article: Chem. Commun 2002, 1226-1227 (pdf)
!-anti-bonding States: (CX)
CH3H
For the latest views, read Alabugin & Zeidan, JACS 2002, 124, 3175 (pdf)
CH3CH3 CH3NH2 CH3OH CH3F
Increasing !"-acceptor capacity
best acceptor
!-anti-bonding States: (CX)
For the latest views, read Alabugin & Zeidan, JACS 2002, 124, 3175 (pdf)
CH3F CH3Cl CH3Br
Your Thoughts on the trend shown below:
The 1,2-Dihaloethanes
X
H H C H H H H C H H
best acceptor
X X
Increasing !"-acceptor capacity
X = Cl; H = + 0.91.3 kcal/mol X = Br; H = + 1.41.8 kcal/mol X = F; H = 0.6-0.9 kcal/mol
D. A. Evans
Stabilized Eclipsed Conformations in Simple Olefins
Butane versus 1-Butene
Me
Chem 206
Simple olefins exhibit unusal conformational properties relative to their saturated counterparts
Propane versus Propene
109 H H H H Me H H H H H 120 CH2
staggered conformation
H C H H
Me H
H H
C H H
Me Me
eclipsed conformation
! G = +4 kcal mol-1
Me
Hybridization change opens up the CCC bond angle
H CH2 C H H H C H CH2 H
staggered conformation
CH2 C H H H
H H
Me CH2 C H
eclipsed conformation
" = 50
! The Propylene Barrier
! G = 0.83 kcal mol-1
"=0
staggered conformation
The Torsional Energy Profile ! = 50 ! = 180
H H H H C H H C Me H Me H C H H C
+2.0 kcal/mol
eclipsed conformation
!=0
H C H H +1.32 kcal H C H H C H Me H +1.33 kcal
New (de)stabilizing effect
H C H Me
H H X H C H H X C H H
stabilizing conjugation between !"CX & #CH
!=0
+0.49 kcal
! = 120
! = 180
Conforms to ab initio (3-21G) values: Wiberg, K. B.; Martin, E. J. Am. Chem. Soc. 1985, 107, 5035.
! Acetaldehyde exhibits a similar conformational bias
O H H H H Me H H O H H H H O Me Me H H O Me
K. Wiberg, JACS 1985, 107, 5035-5041 K. Houk, JACS 1987, 109, 6591-6600
The low-energy conformation in each of above cases is eclipsed
Evans, Duffy, & Ripin
5
Conformational Barriers to Rotation: Olefin A-1,2 Interactions
5
Chem 206
1-butene
H H H C C Me H H
4
2-propen-1-ol
H H H C C OH H H
E (kcal/mol)
E (kcal/mol)
0 -180
-90
90
180
! (Deg)
The Torsional Energy Profile ! = 50
Me H H H C H C H H H C H
0 -180
-90
90
180
! (Deg)
! = 180
H C Me H
The Torsional Energy Profile
H H
H C H C
OH H
! = 60
HO H H C H H C H H
! = 180
!=0
C H Me H C
H H H
+1.32 kcal
H H C H C
Me H H
+1.33 kcal
! = 120
C H C
OH H H
+2.00 kcal
!=0
H
! = 180
H H H
+0.49 kcal
!=0
! = 120
+1.18 kcal
C HO !=0
+0.37 kcal
! = 180
Conforms to ab initio (3-21G) values: Wiberg, K. B.; Martin, E. J. Am. Chem. Soc. 1985, 107, 5035.
Evans, Duffy, & Ripin
5
Conformational Barriers to Rotation: Olefin A-1,2 Interactions-2
Chem 206
2-methyl-1-butene
4
2-methyl-2-propen-1-ol
4
H H H C C Me
H Me
H H H C C OH H Me
E (kcal/mol)
E (kcal/mol)
0 -180
-90
90
180
! (Deg)
The Torsional Energy Profile ! = 180 H
H H C H C Me Me
0 -180
-90
90
180
! (Deg)
The Torsional Energy Profile ! = 180 ! = 60
HO H C H C Me H H H H C H C OH Me
! = 50
Me H H C H C Me H
! = 120
OH
!=0
C H Me !=0 H C
! = 110
H Me H
+1.39 kcal
H H C H C
Me Me H
+2.68 kcal
!=0
H C H HO C
H Me H
+1.16 kcal
H H
+2.01 kcal
Me
C H
C H
+0.06 kcal
+0.21 kcal
! = 180
! = 180
!=0
Evans, Duffy, & Ripin
Conformational Barriers to Rotation: Olefin A-1,3 Interactions
Chem 206
(Z)-2-pentene
4
(Z)-2-buten-1-ol
4
H Me H C C OH
H H
H Me
E (kcal/mol)
E (kcal/mol)
C Me
!
2
0 -180
-90
90
180
0 -180
-90
90
180
! (Deg)
H Me C H Me C H H
! (Deg)
The Torsional Energy Profile !=0
Me C H HO C H H H Me
The Torsional Energy Profile !=0
! = 180
H C H C H OH H
+3.88 kcal
! = 180 ! = 90
Me Me H C H C H H Me H H C H C Me H
+1.44 kcal
Me
! = 120
OH C H C H H
+0.86 kcal
+0.52 kcal
! = 180
H !=0
!=0
! = 180
Values calculated using MM2 (molecular mechanics) force fields via the Macromodel multiconformation search.
Review: Hoffman, R. W. Chem. Rev. 1989, 89, 1841.
Вам также может понравиться
- Chemistry - Harvard's Advanced Organic Chemistry 2003Документ717 страницChemistry - Harvard's Advanced Organic Chemistry 2003ramik100% (23)
- (1908) Mack's Barbers' Guide: A Practical Hand-BookДокумент124 страницы(1908) Mack's Barbers' Guide: A Practical Hand-BookHerbert Hillary Booker 2nd100% (1)
- TSR 9294 DLA3 Dragons RestДокумент78 страницTSR 9294 DLA3 Dragons RestLéo Duarte100% (4)
- 7.1 (149 Marks) : MarkschemeДокумент51 страница7.1 (149 Marks) : MarkschemeSemwezi Enock100% (1)
- Organic ChemistryДокумент187 страницOrganic ChemistryLindayenОценок пока нет
- Notes Lecture 1 Conformational AnalysisДокумент18 страницNotes Lecture 1 Conformational AnalysisDianing Wismarani Putri100% (1)
- Electronic Structure and the Properties of Solids: The Physics of the Chemical BondОт EverandElectronic Structure and the Properties of Solids: The Physics of the Chemical BondРейтинг: 2.5 из 5 звезд2.5/5 (3)
- EL2 - Raise Organic Small RuminantsДокумент62 страницыEL2 - Raise Organic Small RuminantsButch Demayo100% (1)
- EASA Part-66 Module 17 QBДокумент53 страницыEASA Part-66 Module 17 QBFaisal Ahmed Newon80% (5)
- 4.3 (153 Marks) : MarkschemeДокумент62 страницы4.3 (153 Marks) : MarkschemeSemwezi EnockОценок пока нет
- Hyperconjugation, The Anomeric Effect, and More: Chem 206 D. A. EvansДокумент12 страницHyperconjugation, The Anomeric Effect, and More: Chem 206 D. A. Evansomkar9996767Оценок пока нет
- D. A. Evans and F. Michael - An Introduction To Frontier Molecular Orbital Theory-1Документ8 страницD. A. Evans and F. Michael - An Introduction To Frontier Molecular Orbital Theory-1Nuansak3Оценок пока нет
- Practice Questions-Conformational AnalysisДокумент4 страницыPractice Questions-Conformational AnalysisHarry Zgambo100% (1)
- Koehring ManualДокумент56 страницKoehring ManualKyle A. Nolan100% (3)
- Computer Forensics ReportДокумент7 страницComputer Forensics ReportMatias IacobuzioОценок пока нет
- 2400 8560 PR 8010 - A1 HSE Management PlanДокумент34 страницы2400 8560 PR 8010 - A1 HSE Management PlanMohd Musa HashimОценок пока нет
- Photochemistry – 6: Plenary Lectures Presented at the Sixth International Symposium on Photochemistry, Aix-En-Provence, France, 19-23 July, 1976От EverandPhotochemistry – 6: Plenary Lectures Presented at the Sixth International Symposium on Photochemistry, Aix-En-Provence, France, 19-23 July, 1976A. GilbertОценок пока нет
- 100 IELTS Essay Topics For IELTS Writing - My IELTS Classroom BlogДокумент16 страниц100 IELTS Essay Topics For IELTS Writing - My IELTS Classroom BlogtestОценок пока нет
- Absorption Spectra and Chemical Bonding in ComplexesОт EverandAbsorption Spectra and Chemical Bonding in ComplexesРейтинг: 2.5 из 5 звезд2.5/5 (2)
- Chemistry 206 Advanced Organic Chemistry: Chem 206 D. A. EvansДокумент14 страницChemistry 206 Advanced Organic Chemistry: Chem 206 D. A. EvanseraborОценок пока нет
- 12 Pericyclic Rxns 2 PDFДокумент13 страниц12 Pericyclic Rxns 2 PDFPrasanth BitlaОценок пока нет
- 06 Conformational Anal 3Документ13 страниц06 Conformational Anal 3eraborОценок пока нет
- Boger CourseДокумент477 страницBoger CourseharrypoutreurОценок пока нет
- Chemistry 206 Advanced Organic Chemistry: Olefin Addition Reactions: Part-2Документ17 страницChemistry 206 Advanced Organic Chemistry: Olefin Addition Reactions: Part-2eraborОценок пока нет
- Organic Chemistry 2021Документ26 страницOrganic Chemistry 2021xapodi8776Оценок пока нет
- Answer Key: Chemistry 206 First Hour ExaminationДокумент9 страницAnswer Key: Chemistry 206 First Hour Examinationsudipta88Оценок пока нет
- Cuaderno de Trabajo - 2019-2Документ35 страницCuaderno de Trabajo - 2019-2Monica BravoОценок пока нет
- 05 Conformational Anal 2Документ11 страниц05 Conformational Anal 2Swati GautamОценок пока нет
- Lecture 1Документ11 страницLecture 1Fang GaoОценок пока нет
- Isomerism and Stereochemistry: Answers To Worked ExamplesДокумент15 страницIsomerism and Stereochemistry: Answers To Worked ExamplesDana CapbunОценок пока нет
- Chemistry 206 Advanced Organic Chemistry: Chem 206 D. A. EvansДокумент14 страницChemistry 206 Advanced Organic Chemistry: Chem 206 D. A. EvansQuynh TranОценок пока нет
- Hammett Plots2Документ42 страницыHammett Plots2Sankar SasmalОценок пока нет
- Cuaderno de Trabajo - 2019-2Документ35 страницCuaderno de Trabajo - 2019-2Monica BravoОценок пока нет
- 16 Cycloaddition Rxns 1Документ13 страниц16 Cycloaddition Rxns 1Aulia RhamdaniОценок пока нет
- Ferreira Lit 7-17-03Документ14 страницFerreira Lit 7-17-03bocahupiОценок пока нет
- Hyper ConjugationДокумент29 страницHyper ConjugationDargorlethОценок пока нет
- Chapter04 OxidationdДокумент46 страницChapter04 OxidationdWilliam H. BasingerОценок пока нет
- 33 Carbocations 1Документ28 страниц33 Carbocations 1vkrОценок пока нет
- CHM 1321 Assignment #2 - : AnswersДокумент11 страницCHM 1321 Assignment #2 - : AnswersSara YuenОценок пока нет
- Suggested Solutions For Chapter 39: Problem 1Документ18 страницSuggested Solutions For Chapter 39: Problem 1Larry AguirreОценок пока нет
- Additional Electronic Handouts: The Evolution of Stereochemical Models For C O AdditionДокумент54 страницыAdditional Electronic Handouts: The Evolution of Stereochemical Models For C O Additionjames mellaleievОценок пока нет
- AnilineIJQC00 PDFДокумент9 страницAnilineIJQC00 PDFgunjaguptaОценок пока нет
- Reaccion de Eliminacion E y ZДокумент5 страницReaccion de Eliminacion E y ZKarelis GutierrezОценок пока нет
- Ricardo Ugarte, Carlos Bustos, Ignacio Moreno-VillosladaДокумент7 страницRicardo Ugarte, Carlos Bustos, Ignacio Moreno-VillosladaSOCKYОценок пока нет
- Compendium On Problems in Physical-Organic ChemistryДокумент27 страницCompendium On Problems in Physical-Organic ChemistrychemptnkОценок пока нет
- Alkenes and Alkynes: Electrophilic Addition and Pericyclic ReactionsДокумент28 страницAlkenes and Alkynes: Electrophilic Addition and Pericyclic ReactionsRabin ShresthaОценок пока нет
- Supercritical Water Gasification of Biomass Thermodynamic AnalysisДокумент7 страницSupercritical Water Gasification of Biomass Thermodynamic AnalysisLuiz Guilherme SilvaОценок пока нет
- 1 s2.0 0584853971800387 Main PDFДокумент7 страниц1 s2.0 0584853971800387 Main PDFShreetama BhattacharyaОценок пока нет
- Gennari 1992Документ5 страницGennari 1992Hữu Nhân Hạ Mục Linh TửОценок пока нет
- Enu Tour1 TaskДокумент9 страницEnu Tour1 TaskĐinh Đại VũОценок пока нет
- Chapter 12Документ23 страницыChapter 12Hải NguyễnОценок пока нет
- Allylic StrainДокумент18 страницAllylic StrainRahn NaОценок пока нет
- Thermodynamics of Metallic SolutionДокумент34 страницыThermodynamics of Metallic SolutionJaswant Singh ChauhanОценок пока нет
- Aromatic-Aromatic Interactions Free Energy Profiles For The Benzene Dimer in Water Chloroform and Liquid BenzeneДокумент7 страницAromatic-Aromatic Interactions Free Energy Profiles For The Benzene Dimer in Water Chloroform and Liquid BenzeneEsteban ArayaОценок пока нет
- Thermal Isomerization of Cis and TransДокумент8 страницThermal Isomerization of Cis and Transa9rinaОценок пока нет
- Vrouw, Mar 2011Документ4 страницыVrouw, Mar 2011emediageОценок пока нет
- Organic Chemistry NotesДокумент3 страницыOrganic Chemistry NotesHalimaОценок пока нет
- Problems On Named ReactionsДокумент103 страницыProblems On Named ReactionsBapu ThoratОценок пока нет
- Anomeric EffectДокумент2 страницыAnomeric EffectBen Duncan Málaga EspichánОценок пока нет
- Bi FunctionalДокумент36 страницBi FunctionalchidambaramrОценок пока нет
- The Most Well-Known Rearrangements in Organic Chemistry at HandДокумент32 страницыThe Most Well-Known Rearrangements in Organic Chemistry at HandAnkit JagetiaОценок пока нет
- Urea DegradationДокумент32 страницыUrea DegradationGarret LoganОценок пока нет
- Coursesaver (Chad) College Physics OutlinesДокумент40 страницCoursesaver (Chad) College Physics Outlinescalong558Оценок пока нет
- Stereoelectronic Effects: A Bridge Between Structure and ReactivityОт EverandStereoelectronic Effects: A Bridge Between Structure and ReactivityОценок пока нет
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsОт EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsОценок пока нет
- Ion Association in Proton Transfer Reactions: Use of ESR for the Quantitative Determination of Gas Phase Atom and Radical ConcentrationsОт EverandIon Association in Proton Transfer Reactions: Use of ESR for the Quantitative Determination of Gas Phase Atom and Radical ConcentrationsОценок пока нет
- Plasma Chemistry: International Symposium on Plasma ChemistryОт EverandPlasma Chemistry: International Symposium on Plasma ChemistryD. E. JensenОценок пока нет
- Preparatory Newsletter No 3 2013Документ22 страницыPreparatory Newsletter No 3 2013SJC AdminОценок пока нет
- Paper 3 FrinqДокумент4 страницыPaper 3 Frinqapi-301975170Оценок пока нет
- Amlodipine Besylate Drug StudyДокумент2 страницыAmlodipine Besylate Drug StudyJonieP84Оценок пока нет
- NM Integrative Wellness MRC Public Health Acupuncture JITTДокумент40 страницNM Integrative Wellness MRC Public Health Acupuncture JITTPrince DhillonОценок пока нет
- Good Data Won't Guarantee Good DecisionsДокумент3 страницыGood Data Won't Guarantee Good DecisionsAditya SharmaОценок пока нет
- Unit 9 TelephoningДокумент14 страницUnit 9 TelephoningDaniela DanilovОценок пока нет
- A. Erfurth, P. Hoff. Mad Scenes in Early 19th-Century Opera PDFДокумент4 страницыA. Erfurth, P. Hoff. Mad Scenes in Early 19th-Century Opera PDFbiarrodОценок пока нет
- Pre-Qin Philosophers and ThinkersДокумент22 страницыPre-Qin Philosophers and ThinkersHelder JorgeОценок пока нет
- Emerging and Less Common Viral Encephalitides - Chapter 91Документ34 страницыEmerging and Less Common Viral Encephalitides - Chapter 91Victro ChongОценок пока нет
- Demystifying The Diagnosis and Classification of Lymphoma - Gabriel C. Caponetti, Adam BaggДокумент6 страницDemystifying The Diagnosis and Classification of Lymphoma - Gabriel C. Caponetti, Adam BaggEddie CaptainОценок пока нет
- Dispersion Relation of Electromagnetic WavesДокумент2 страницыDispersion Relation of Electromagnetic WavesFidel SouzaОценок пока нет
- E Catalog YooilДокумент10 страницE Catalog Yooilom jangidОценок пока нет
- Rastriya Swayamsewak SanghДокумент60 страницRastriya Swayamsewak SanghRangam Trivedi100% (3)
- DRUG STUDY (Erythromycin)Документ3 страницыDRUG STUDY (Erythromycin)Avianna CalliopeОценок пока нет
- Minuto hd8761Документ64 страницыMinuto hd8761Eugen Vicentiu StricatuОценок пока нет
- Azure Arc DoccumentДокумент143 страницыAzure Arc Doccumentg.jithendarОценок пока нет
- Minglana-Mitch-T-Answers in Long QuizДокумент9 страницMinglana-Mitch-T-Answers in Long QuizMitch MinglanaОценок пока нет
- Evelyn Arizpe - Teresa Colomer - Carmen Martínez-Roldán - Visual Journeys Through Wordless Narratives - An International Inquiry With Immigrant Children and The Arrival-Bloomsbury Academic (2014)Документ290 страницEvelyn Arizpe - Teresa Colomer - Carmen Martínez-Roldán - Visual Journeys Through Wordless Narratives - An International Inquiry With Immigrant Children and The Arrival-Bloomsbury Academic (2014)Lucia QuirogaОценок пока нет
- Bruner, Jerome - The Growth of MindДокумент11 страницBruner, Jerome - The Growth of MindTalia Tijero100% (1)
- Note 15-Feb-2023Документ4 страницыNote 15-Feb-2023Oliver ScissorsОценок пока нет
- Designing The Workplace For CollaborationДокумент17 страницDesigning The Workplace For Collaborationmas zak danielОценок пока нет
- Chapter 3Документ26 страницChapter 3Francis Anthony CataniagОценок пока нет