Академический Документы
Профессиональный Документы
Культура Документы
Ionic Equilibria and Resting Membrane Potential Lecture #11, BB, Chap. 6, Pgs. 147 - 156
Загружено:
George Livingston GrahamОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Ionic Equilibria and Resting Membrane Potential Lecture #11, BB, Chap. 6, Pgs. 147 - 156
Загружено:
George Livingston GrahamАвторское право:
Доступные форматы
Ionic Equilibria and Resting Membrane Potential Lecture #11, BB, Chap. 6, pgs.
147 - 156
I. Introduction A. membrane electrical properties enable: 1. cellular communication 2. muscle contraction 3. hormonal secretion 4. maintenance of ionic constituency
B. early experiments with electrical properties of cells
B. determination of cell potentials
II. Resting Membrane Potential A. diffusion potential 1. active transport establishes ionic concentration gradients 2. ions diffuse across membrane based upon selective permeability 3. results in small potential difference (mV) across membrane
4. role of active transport and ion diffusion a. Na-K ATPase establishes ion gradients i. [Na+]i < [Na+]o ii. [K+]i > [K+]o b. K+ diffuses out of cell c. Na+ much less permeable
Na+
Na+
Na+
K+
K+
d. net K+ efflux establishes potential gradient i. K+ influx occurs due to potential gradient ii. influx continues until: (a) K efflux due to [ ] gradient = K influx due to potential gradient (b) termed electrochemical equilibrium
Na+
Na+
Na+
+ -+ chemical - + - + potential +
K+
K+
B. Nernst Equation 1. potential difference across membrane @ electrochemical equilibrium 2. specific for ion being considered (e.g. potassium)
EK = - zF ln
RT ___
[K+]i
____
[K+] o
Where: K = diffusion potential for potassium R = gas constant (.082L-atm/mole-Ko) z = ion charge F = Faraday constant (96,500 coulombs/g charge) T = temperature (Kelvin, Co + 273)
3. In a mammalian system, the Nernst equation is often simplified: a. 37o C. (body temperature) b. monovalent ions c. convert ln to log (base of 10)
+]i [K ___ EK = - 60log + mV [K ]
o
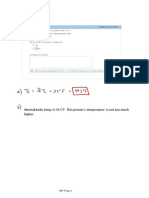
4. Example: if [K+]o = 10 mM and [K+]i = 100 mM, then:
+]i [K ___ = 10; log of 10 = 1 [K+]
o
EK = -60(1) = - 60 mv
C. reversal potential 1. ion currents vs. membrane potential a. Vm = Ex - no net ion (x) current b. Vm > Ex (more negative) net inward current c. Vm < Ex (more positive) net outward current 2. Vrev = potential at which the current reverses
D. resting membrane potential (Em) 1. introduction a. property of all living cells b. result of active transport & selective permeability
c. Em results from EC equilibrium of all of ions i. Na+, K+ and Cl- all contribute to Em ii. however, effect upon Em, dependent upon permeability
2. Chord (Goldman-Hodgekin-Katz) Equation
o ________ o_________________ ___ Em = 60 log __ _______ + +
K Na
PK[K+] + PNa[Na+] + PCl[Cl-]i P [K ]i + P [Na ]i + P [Cl-]
Cl
a. Since: PK >> PNa : i. Em K diffusion potential ii. Em differs between of cell types (1) neural -90 mV (2) muscles - 50 to - 70 mV (3) epithelia few mV iii. dependent on relative ion permeability
3. Since Vm is essentially a potassium diffusion potential changes in [K+] can markedly effect resting membrane potential
E. Na-K ATPase is an electrogenic transporter 1. active transport contributes directly to Vm 2. cations are unequally translocated across membrane a. 2:3 (2 K influx:3 Na efflux) b. net efflux of charge (outside positive, relative to inside) 3. magnitude a. depends on membrane b. approx 2 - 5 mV of Vm due to electrogenic activity
III. Gibbs Donnan Equilibrium (BB, page 133 - 134)
A. introduction
1. cytoplasm contains: a. both charged & non-charged solute b. both permeant & non-permeant solute 2. G-D equilibrium describes steady state properties of this mixture
B. consider two solutions separated by a semipermeable membrane
1. initial conditions:
2. membrane permeable to K+ and Cl- but not Ya. A and B isosmotic and electrically neutral
2. equilibrium conditions
a. b. c. d.
Cl- diffuses into A, down [ ] gradient K+ diffuses into A to maintain electroneutrality at equilibrium permeant ions (K and Cl) are both EC equilbrium in a typical cell this contributes -10 mV to the cell potential
3. Gibbs Donnan effects upon osmotic pressure a. ion shift has resulted in a disparity of solute concentration b. water diffuses from B to A to equalize osmolality
4. physiological application a. proteins negatively charged at biological pH b. confined by cell membranes w/i compartments c. result is shift in non-confined ions (& H2O) across membrane d. ~ 5% shift of confined solute in mammals, discussed in body fluid balance lecture e. elevates resting membrane potential and cell volume
Вам также может понравиться
- Copeland Discus Digital Compressors Coresense Technology en 2884258Документ2 страницыCopeland Discus Digital Compressors Coresense Technology en 2884258Roberto CastilloОценок пока нет
- Bioelectricity 1 Equilibrium & MPДокумент4 страницыBioelectricity 1 Equilibrium & MPZiyuan CaiОценок пока нет
- Lec. 11new - 05 - Membrane PotentialsДокумент33 страницыLec. 11new - 05 - Membrane PotentialsDivine SundayОценок пока нет
- Electrical Properties of Cell MembraneДокумент32 страницыElectrical Properties of Cell Membranemihaela irofte75% (4)
- Membrane PotentialsДокумент24 страницыMembrane PotentialsJeff ParkОценок пока нет
- Biology 4822: Resting Membrane Potential Reviewed and ContinuedДокумент30 страницBiology 4822: Resting Membrane Potential Reviewed and ContinuedKimberly Parton BolinОценок пока нет
- NROSCI 1012 - Lecture 10Документ4 страницыNROSCI 1012 - Lecture 10HonzaОценок пока нет
- NROSCI 1012 - Lecture 13Документ4 страницыNROSCI 1012 - Lecture 13HonzaОценок пока нет
- 6) 2020 ElectrochemistryДокумент14 страниц6) 2020 ElectrochemistryFaizan AnsariОценок пока нет
- Questions ExplanationДокумент17 страницQuestions ExplanationnomintmОценок пока нет
- Neurobiology 15-BIOL-540 Winter, 2012Документ63 страницыNeurobiology 15-BIOL-540 Winter, 2012bcastle1Оценок пока нет
- NROSCI 1012 - Lecture 9Документ4 страницыNROSCI 1012 - Lecture 9HonzaОценок пока нет
- 03membrane PotentialДокумент18 страниц03membrane PotentialJaydave PatelОценок пока нет
- Electrochemical EquilibriumДокумент13 страницElectrochemical EquilibriumChelsea MartinezОценок пока нет
- HBY 531 Medical Physiology 2003 Lecture Exam 1Документ6 страницHBY 531 Medical Physiology 2003 Lecture Exam 1Kathryn GerberОценок пока нет
- Electricity & Patch ClampДокумент8 страницElectricity & Patch ClampZiyuan CaiОценок пока нет
- 11Документ55 страниц11Shoniqua JohnsonОценок пока нет
- Questions - Answers Bank Class - Xii Subject - Chemistry UNIT-3 (Electrochemistry)Документ6 страницQuestions - Answers Bank Class - Xii Subject - Chemistry UNIT-3 (Electrochemistry)Abhay BharadwajОценок пока нет
- 4.07 Ions in Aqueous Solution: (5 Points)Документ9 страниц4.07 Ions in Aqueous Solution: (5 Points)NadunKodikaraОценок пока нет
- NROSCI 1012 - Lecture 11Документ4 страницыNROSCI 1012 - Lecture 11HonzaОценок пока нет
- Lesson 1 Electric Charge Coulombs Law Electric Fields and Electric Flux2 PDFДокумент10 страницLesson 1 Electric Charge Coulombs Law Electric Fields and Electric Flux2 PDFAjzОценок пока нет
- Electric ChargeДокумент10 страницElectric ChargeAmethyst0% (1)
- Britney Patterson - Six Week TestДокумент10 страницBritney Patterson - Six Week TestBritney PattersonОценок пока нет
- Bio Instrum OriginbiopДокумент49 страницBio Instrum OriginbiopCALEB DAVID ROMERO MERCADOОценок пока нет
- Leakiness: Lab 2: Neural SignallingДокумент2 страницыLeakiness: Lab 2: Neural SignallingAfroza ShoilyОценок пока нет
- ElectrochemistryДокумент9 страницElectrochemistryrandom idОценок пока нет
- Negative Negative: Chapter Two: Electrical Signals in Nerve CellsДокумент6 страницNegative Negative: Chapter Two: Electrical Signals in Nerve CellsStacy BrenesОценок пока нет
- Engg Chemistry PDFДокумент201 страницаEngg Chemistry PDFShamawn Muktadeer ShovonОценок пока нет
- Bioelectric Potential: Dr. Anju Jha Mbbs. Md. PGDMCHДокумент63 страницыBioelectric Potential: Dr. Anju Jha Mbbs. Md. PGDMCHzohaibОценок пока нет
- 2 Mem TransДокумент6 страниц2 Mem TransPhuong DoanОценок пока нет
- Electrostatics 1Документ108 страницElectrostatics 1Raman KalraОценок пока нет
- 03.resting Potential & Action PotentialДокумент122 страницы03.resting Potential & Action Potentialapi-19916399Оценок пока нет
- Electricity 43pgДокумент43 страницыElectricity 43pgbalrajsiwachОценок пока нет
- AP Physics B Notes - ElectrostaticsДокумент8 страницAP Physics B Notes - ElectrostaticsAndy HeОценок пока нет
- 2-1 r18 - Electronic Devices & CircuitsДокумент163 страницы2-1 r18 - Electronic Devices & Circuits20261A0448 SREYA SОценок пока нет
- @sachin - Sir - Physics: Version 2.OДокумент9 страниц@sachin - Sir - Physics: Version 2.OPrakharОценок пока нет
- Microwave Semiconductor Device Technologies 4. Energy Bands and Charge CarrierДокумент11 страницMicrowave Semiconductor Device Technologies 4. Energy Bands and Charge Carriersushil4056Оценок пока нет
- RestingMembranePotential RevisionДокумент5 страницRestingMembranePotential RevisionshaОценок пока нет
- NeurophysiologyДокумент117 страницNeurophysiologyRachel CajilesОценок пока нет
- Donnanova Ravnoteya I Membranski PotencijaliДокумент21 страницаDonnanova Ravnoteya I Membranski PotencijaliPredrag DjurdjevicОценок пока нет
- Neurobiology, Physiology, and BehaviorДокумент9 страницNeurobiology, Physiology, and BehaviorcindyucdОценок пока нет
- Electrostatics (E)Документ128 страницElectrostatics (E)Krishna RОценок пока нет
- Appendix C - Hodgkin-Huxley EquationsДокумент5 страницAppendix C - Hodgkin-Huxley EquationsBoris ShalomovОценок пока нет
- Neurons Resting PotentialДокумент5 страницNeurons Resting PotentialEstelle HagenОценок пока нет
- Biomedical Instrumentation I: Lecture-5: The Origin of BiopotentialsДокумент35 страницBiomedical Instrumentation I: Lecture-5: The Origin of BiopotentialsNitin PrajapatiОценок пока нет
- S S E S: PhysicsДокумент4 страницыS S E S: PhysicsRAJ STUDY WIZARDОценок пока нет
- ELECTROCHEMISTRYДокумент21 страницаELECTROCHEMISTRYRahul PrajapatiОценок пока нет
- 12 ChemistryДокумент3 страницы12 ChemistryShudufhadzo PrettyОценок пока нет
- The Hodgkin-Huxley ModelДокумент7 страницThe Hodgkin-Huxley ModelLike Hell I'm Telling YouОценок пока нет
- Lec 2 - Measurement ModalitiesДокумент24 страницыLec 2 - Measurement ModalitiesMohsin SarfrazОценок пока нет
- Electro ChemistryДокумент34 страницыElectro ChemistryFam IlyОценок пока нет
- Electric Charge and FieldДокумент35 страницElectric Charge and FieldIndraneil SenguptaОценок пока нет
- Lecure-5 The Origin of Biopotentials - 2Документ34 страницыLecure-5 The Origin of Biopotentials - 2Noor Ahmed100% (1)
- Chem 127 Trans 2 Bulk ElectrolysisДокумент22 страницыChem 127 Trans 2 Bulk ElectrolysisBeam CanoОценок пока нет
- P 62Документ25 страницP 62JohnnardBelenОценок пока нет
- MessageДокумент3 страницыMessageabdulrahmanmohammad242kbsОценок пока нет
- DR Khatatbeh 2 Lec 5Документ29 страницDR Khatatbeh 2 Lec 5api-249972919Оценок пока нет
- Volume 2 Interfacial Kinetics and Mass TransportДокумент526 страницVolume 2 Interfacial Kinetics and Mass TransportEugenОценок пока нет
- Current ElectricityДокумент112 страницCurrent Electricitymkg_met2391Оценок пока нет
- Bioelectrochemistry of Biomembranes and Biomimetic MembranesОт EverandBioelectrochemistry of Biomembranes and Biomimetic MembranesОценок пока нет
- Modern Electrical Installation for Craft StudentsОт EverandModern Electrical Installation for Craft StudentsРейтинг: 4.5 из 5 звезд4.5/5 (4)
- Fluorescence Experiment DuДокумент1 страницаFluorescence Experiment DuGeorge Livingston GrahamОценок пока нет
- Ch03 LectureДокумент98 страницCh03 LectureGeorge Livingston GrahamОценок пока нет
- Ch05 LectureДокумент58 страницCh05 LectureGeorge Livingston GrahamОценок пока нет
- Generation and Conduction of Action Potentials: A. Action Potential CharacteristicsДокумент17 страницGeneration and Conduction of Action Potentials: A. Action Potential CharacteristicsGeorge Livingston GrahamОценок пока нет
- Appendicular System MTT, Chap. 11:284 - 326Документ15 страницAppendicular System MTT, Chap. 11:284 - 326George Livingston GrahamОценок пока нет
- HW1 SolnsДокумент26 страницHW1 SolnsGeorge Livingston GrahamОценок пока нет
- HW 9 SolnsДокумент25 страницHW 9 SolnsGeorge Livingston GrahamОценок пока нет
- Blacboard For StudentsДокумент1 страницаBlacboard For StudentsGeorge Livingston GrahamОценок пока нет
- HW 8 SolnsДокумент20 страницHW 8 SolnsGeorge Livingston GrahamОценок пока нет
- DesktopДокумент2 страницыDesktopGeorge Livingston GrahamОценок пока нет
- Control of Gene Regulation in EukaryotesДокумент39 страницControl of Gene Regulation in EukaryotesGeorge Livingston GrahamОценок пока нет
- Research Proposal TransformerДокумент3 страницыResearch Proposal Transformersohalder1026Оценок пока нет
- Relayoperationprinciples 141126065914 Conversion Gate01Документ43 страницыRelayoperationprinciples 141126065914 Conversion Gate01kenlavie2Оценок пока нет
- 2 - EE - Intro - Electronics Pg. 28-41 Op Amp-Merged PDFДокумент402 страницы2 - EE - Intro - Electronics Pg. 28-41 Op Amp-Merged PDFAdelin IonutОценок пока нет
- Alp - Sizer InfoДокумент13 страницAlp - Sizer InfoLê Quang DuyОценок пока нет
- Ashikur Rahman 011172081 B: Name: IDДокумент13 страницAshikur Rahman 011172081 B: Name: IDAshikОценок пока нет
- Corrosion Properties of Copper Nickel Alloys in Chlorinated Sea WaterДокумент14 страницCorrosion Properties of Copper Nickel Alloys in Chlorinated Sea WaterArunОценок пока нет
- An Empirical Study On The Nexus Between The Emotional Intelligence of Top Managers and Their Assessment of Intellectual CapitalДокумент30 страницAn Empirical Study On The Nexus Between The Emotional Intelligence of Top Managers and Their Assessment of Intellectual Capitalmaher76Оценок пока нет
- Differentiation11 21Документ75 страницDifferentiation11 21Maryam ShahidОценок пока нет
- 1575 Tania SultanaДокумент10 страниц1575 Tania SultanaTania SultanaОценок пока нет
- CORE JAVA (3-0-0) Module - I (10 Hours)Документ3 страницыCORE JAVA (3-0-0) Module - I (10 Hours)Rupak BhuyanОценок пока нет
- Analytical Investigation of Entropy Production With Convective Heat Transfer in Pressure Driven Flow of A Generalised Newtonian FluidДокумент30 страницAnalytical Investigation of Entropy Production With Convective Heat Transfer in Pressure Driven Flow of A Generalised Newtonian FluidUğur DemirОценок пока нет
- Prob AnswersДокумент4 страницыProb AnswersDaniel KirovОценок пока нет
- Astron: MFL Testing Procedure For Tank FloorДокумент16 страницAstron: MFL Testing Procedure For Tank FloorleonciomavarezОценок пока нет
- Buffer SolutionДокумент6 страницBuffer SolutionAdrija MandalОценок пока нет
- Carbon Compounds: Standard/ Class/ Grade - 10 SSC, CBSE - 8 ICSEДокумент53 страницыCarbon Compounds: Standard/ Class/ Grade - 10 SSC, CBSE - 8 ICSEsaintEmОценок пока нет
- Datasheet Cpu 416-2Документ13 страницDatasheet Cpu 416-2Danu MaldinoОценок пока нет
- Wasif CVДокумент2 страницыWasif CVTalha BaigОценок пока нет
- Intro To PVTДокумент19 страницIntro To PVTFernando OlaveoОценок пока нет
- Class Problems Sentences 13 The DДокумент20 страницClass Problems Sentences 13 The DKnowledgeIsTruePowerОценок пока нет
- Ali Math Competition 3 English Reference SolutionsДокумент11 страницAli Math Competition 3 English Reference SolutionsJEREMIAH ITCHAGBEОценок пока нет
- NOJA 520 05 SCADA Interface Description PDFДокумент24 страницыNOJA 520 05 SCADA Interface Description PDFsergio torrez vargasОценок пока нет
- Electronic Devices & Practice: InstructorДокумент23 страницыElectronic Devices & Practice: Instructorjavaid musaОценок пока нет
- Instruction Manual B-Tronic SystemДокумент35 страницInstruction Manual B-Tronic SystemYipper ShnipperОценок пока нет
- Surveying PDFДокумент215 страницSurveying PDFShaira Mae Cañedo100% (1)
- CTX 310 Communication With Fast Ethernet Board V2 (Fanuc)Документ34 страницыCTX 310 Communication With Fast Ethernet Board V2 (Fanuc)iveОценок пока нет
- Magnetism: Teacher Notes and Answers 19 MagnetismДокумент3 страницыMagnetism: Teacher Notes and Answers 19 Magnetismmahsan abbas100% (1)
- A Generic Circular BufferДокумент3 страницыA Generic Circular BufferSatish KumarОценок пока нет
- 1 Logic GatesДокумент4 страницы1 Logic GatesdassonyОценок пока нет
- Weebly ReportДокумент15 страницWeebly Reportapi-316004735Оценок пока нет