Академический Документы
Профессиональный Документы
Культура Документы
Mercury Lead Arsenic Cadmium Toxicity
Загружено:
SunilОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Mercury Lead Arsenic Cadmium Toxicity
Загружено:
SunilАвторское право:
Доступные форматы
Metal poisoning mercury,
lead, cadmium
Lecture No. 9
Copyright Mgr. Zuzana irok, PhD.
Mercury - Hg
Only metal which is liquid at room temperature
Vapours are much more dangerous
Both organic and inorganic compounds, all toxic
Sources: earths crust and industry
Cummulation in water environment
Water microorganisms transform pure mercury into
methylmercury most common source of poisonings
incorporation into food chain (fish)
Other cases of intoxication usually occupational, mistakes
Iraq (wheat seed with antifungal mercury compound
exchanged for food), Minamata disease (fishermen)
Inorganic mercury compounds:
HgCl
2
, Hg(CN)
2
, Hg(NO
3
)
2
Transported bound to blood peptides
Mercury binds with covalent bond to -SH,
-COOH and His
This influences function of many enzymes
and cell processes
Water soluble salts moreover coagulate
peptides and are corrosive
They damage kidney tubules and GIT mucosa
Organic mercury compounds:
Methoxyethylmercury and arylmercury compounds release
mercuric ions act like inorganic compounds
Methyl- and ethylmercury firm bond, whole compound toxic
Bind with Cys and SH, block enzymes, destroy
haematoencephalic (blood-brain) barrier, increase permeability
Don't have corrosive effect on mucosas
In blood transported bound to erythrocytes
They have high affinity to neural tissue
Cummulate also in liver and kidney
Cross the placenta and have fetotoxic effect Excretion to faeces,
urine, milk, sweat, saliva
Deposition in hair and skin, excretion very slow
Clinical signs:
- in dogs, cats and young cattle stimulation of CNS
- in cattle, pigs and poultry depression of CNS
in dogs: blindness, involuntary chewing
in cats: weakness, ataxia, rigidity of hind limbs, convulsions, glass-
like gaze, miaowing, hypersalivation
in calves: ataxia, limping, stumbling, clonuses of eyelids and ears,
hypersensitivity, malfunction of swallowing, disturbance of
vision, convulsions, opistotonus, coma, death
in cattle: anorexia, loss of weight, weakness, loss of coordination,
salivation, lacrimation, diarrhoea and colic, loss of teeth, swollen
lymphatic nodes, disturbances in EKG, cough, dyspnoe, eczema,
hyperkeratosis. Convulsions very rare
in pigs: inappetence, colic, diarrhoea, hypermotility, tremors, loss
of coordination, they are hunched, paralysis of hind limbs
in poultry: ataxia, loss of coordination, pale cere and lobes
Acute intoxication: more often in inorganic poisoning,
mainly GIT signs, oliguria, uraemia, typical mercury bluish
gum line, decrease of blood pressure, CNS disturbances
Chronic intoxication: typical for methylmercury, not GIT
signs, damage of CNS, kidneys, again bluish margin on
gingiva, loss of teeth, tremor
Pathoanatomical examination:
- reduction of cerebellum, leptomeningitis in cats,
congestion and haemorhagia in brain, stomatitis, enteritis,
petechias
- histology swollen axons, demyelinisation, vacuolisation
on neurons, hyperplasia of epithelium
Treatment: chelate agents penicilamin (not dimercaprol
or EDTA!), vitamin E and Se antioxidants, spironolacton
blocks binding of organic mercury compounds to
erythrocytes in acute poisoning
Lead - Pb
Soft, grey metal
Known since ancient times
Absolutely abiogenic to organisms
Used in pipes, tetraethyl-lead as a petrol
additive, red-lead (minium) primer paintings
Most poisonings in cattle (lead paintings,
batteries in silage)
Both inorganic and organic compounds like in
mercury, different characteristics
Absorption and elimination:
- Inorganic lead: toxic after digestion
- Organic lead: toxic after skin contact, digestion,
inhalation
- Resorption is promoted by calcium, iron and fats in food,
higher in young animals
- Transported bound to erythrocytes (90 %)
- High deposition in tissues first in liver, then
redistributed to bones (inorg.), kidneys, muscles and
hair
- Bone-lead becomes mobilized through pregnancy or
fracture healing
- Excretion via bile to faeces, also to urine and milk
- Inorganic compounds acummulate more and elimination
is very slow, organic compounds excreted much quicker
- Normal blood level of lead cca 0,1 mg/kg, above 0,4 it is
considered as toxic
Mechanism of action:
- Inorganic lead:
- Disturbs saccharide metabolism, metabolism of haem -
inhibits Ala-D (Delta-aminolevulinic acid dehydratase)
increased concentration of aminolevulinic acid in urine
- The toxicity comes from its ability to mimic other
biologically important metals - calcium, iron and zinc
and to interact with proteins
- Organic lead:
- interferes with excitatory neurotransmission by
glutamate
- it is a potent inhibitor of the NMDA receptor, a protein
playing an important role in brain development and
cognition (also in development of schizophrenia)
- doesn't influence synthesis of haem much
Clinical signs of intoxication:
- Acute intoxication:
- from 12 92 hours after absorption
- apathy, atonia of rumen, anorexia, CNS disturbances
tremor of head, neck, loss of coordination, salivation,
gnashing of teeth, aggressiveness, convulsions,
blindness, death due to respiration collapse
- Subacute intoxication:
- similar symptoms, but more severe GIT damage,
changing of constipation and severe diarrhoea, strong
colic pains, mydriasis, opistotonus
- Chronic intoxication:
- inappetence, anorexia, paresis, paralysis of n. recurens
in horse whistling, typical grey gum line, CNS
disturbances
Pathological examination:
- Typical smell from cadaver, petechias
- Green-grey colour of muscles
- Corrosive changes on GIT mucosa
- Dystrophic kidneys
Diagnostics:
- Samples of blood, urine, muscle, liver etc.
- Assessment of lead in these samples + assessment of
aminolevulinic acid in blood and urine
Treatment:
- Usually only in pets
- Gastrolavage, administration of activated charcoal, laxatives
MgSO
4
- Chelating agents EDTA, penicilamin
Cadmium - Cd
No constructive purpose in the body
Extremely toxic even in low concentrations,
accumulates in organisms and ecosystems
Chemical properties similar to zinc exchange in an
organism (also can replace Cu, Fe, Ca)
Sources: earth crust, fossil fuels, plastic materials
industry, electronic industry, tobacco fume
Absorption after digestion or less by inhalation (but
here better bioavailability)
The first documented case of mass cadmium poisoning
in the world - in Toyama Prefecture, Japan in 1950
Itai-Itai disease (river polluted with waste from factory,
water used on rice fields poisoning from rice)
- In blood transported bound to proteins (formation
of complexes), in higher concentrations bound to
erythrocytes
- Deposition in liver, kidneys and gonads
Mechanism of action:
- Inhibition of many enzymes, antagonist to many
metals Zn, Cu, Ca, Fe
- Disturbance of cholecalciferol production, thus
influences Ca metabolism
- Inhibition of specific hydrolase in testis affects
activity of gonads
- Formation of complexes, which are digested in
kidney release of Cd damage
- Xenoestrogennic effect
Clinical signs:
- Acute exposure:
- Cadmium fumes may cause flu like symptoms
including chills, fever, and muscle ache
- More severe exposures can cause tracheo-
bronchitis, pneumonitis, and pulmonary oedema.
Symptoms of inflammation may start hours after
the exposure and include cough, dryness and
irritation of the nose and throat, headache,
dizziness, weakness, fever, chills, and chest pain.
- Ingestion of any significant amount of cadmium
causes immediate poisoning and damage to the
liver and the kidneys. Also CNS disturbances occur
and changes in blood count
- Chronic exposure:
- Osteomalacia, osteoporosis disturbance of vitamin
D and calcium metabolism
- Pain in the joints and the back, and also increases
the risk of fractures. In extreme cases of cadmium
poisoning, the mere body weight causes a fracture
- The kidneys lose their function to remove acids
from the blood. The kidney damage inflicted by
cadmium poisoning is irreversible and does not heal
over time.
- Gout, a form of arthritis due to the accumulation of
uric acid crystals in the joints (hyperuricemia).
- Some patients may lose their sense of smell
(anosmia)
- Damage of gonads, suspected carcinogen tumours
of testes
Pathological examination:
- gastritis, enteritis, nephritis, stomatitis,
degeneration of liver, necrosis on testes
Treatment :
- Chelating agents EDTA only partially effective
- Administration of calcium
More info:
http://www.ilo.org/encyclopedia/?doc&nd=857200247&
nh=0
http://enhs.umn.edu/hazards/hazardssite/mercury/merc
healtheffects.html
http://www.niehs.nih.gov/
http://www.ra.mahidol.ac.th/journal/index.php?comma
nd=preview&selvol=27&selno=1&selids=156
http://www.nsc.org/library/facts/lead.htm
http://www.calpoison.org/public/lead.html
http://www.lead.org.au/au.html
http://www.atsdr.cdc.gov/toxprofiles/tp5.pdf
http://www.portfolio.mvm.ed.ac.uk/studentwebs/sessio
n2/group29/introtox.htm
Introduction
Liquid metal
Used in dentistry Dental amalgam
Acc to ADA > 100 million silver fillings/ year
History
> 2000 years in preparations such as diuretics,
anti-bacterial ointments, laxatives and skin
ointments.
original amalgamation process was
demonstrated by a chemist in France
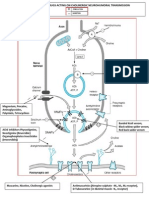
Toxic Effects of Mercury Exposure
Mercury poisoning, also known as
mercurialism, is the phenomenon of
toxication by contact with mercury.
Pure elemental mercury
is a cumulative
heavy-metal poison
moderately absorbed through the skin,
rather poorly absorbed through the
gastrointestinal tract
readily absorbed as vapor through the lungs.
2 Biggest Hg poisonings
Mad Hatter- during the
industrial revolution, hat-
makers used mercury nitrate to
soften fur used as lining in hats.
Toxic effected brains causing
mental instability.
Japan - 1952, chemical co.
dumped Hg into Minamata
harbor; residents of local
fishing villages contaminated;
100s affected and 68 died
Hazardous!!
DANGER! CORROSIVE. CAUSES BURNS TO
SKIN, EYES, AND RESPIRATORY TRACT.
MAY BE FATAL IF SWALLOWED OR
INHALED. HARMFUL IF ABSORBED
THROUGH SKIN. AFFECTS THE KIDNEYS
AND CENTRAL NERVOUS SYSTEM. MAY
CAUSE ALLERGIC SKIN REACTION. (MSDS
2008)
Toxicity
Compounds of
mercury tend to be
much more toxic than
the element itself, and
organic compounds of
mercury are often
extremely toxic.
Dimethylmercury, for
example, is a potent
neurotoxin that is
lethal in amounts of a
fraction of a milliliter.
Mercury damages
the central nervous
system, endocrine
system, kidneys, and
other organs, mouth,
gums, and teeth.
Exposure over long
periods of time or
heavy exposure to
mercury vapor can
result in brain
damage and death.
Mercury and its
compounds are
particularly toxic to
fetuses and infants.
Is it reversible?
Some of the toxic effects of mercury are reversible,
either through specific therapy or through natural
elimination of the metal after exposure has been
discontinued.
However, heavy or prolonged exposure can do
irreversible damage, particularly in fetuses, infants,
and young children. Exposure to certain highly toxic
compounds of mercury such as dimethylmercury can
be fatal within hours or less.
Case Studies
From 1932 to 1968 methyl mercury was released
into the sea around the city of Minamata, Japan.
The toxin bioaccumulated in fish, which when
eaten by the local population caused the largest
case of mercury poisoning known
Minamata disease caused the deaths of over 1000
people and permanently disabled a great many
more.
Another case of widespread mercury
poisoning occurred in rural Iraq in 1971-
1972, when grain treated with a
methylmercury-based fungicide was used
by the rural population to make bread.
Case Studies
In December 1997, a
chemistry professor,
Karen Wetterhahn, at
Dartmouth College was
contaminated with
dimethylmercury when
she spilled a drop on her
latex glove.
China's first emperor of
unified China, Qin Shi
Huang Di, was driven
insane and killed by
mercury pills intended to
give him eternal life.
Symptoms
1. Psychological disturbances
2. Oral Cavity problems
3. Digestive tract problems
4. Cardiovascular problems
5. Respiratory problems
6. Neurological Problems
History
In 1833, two English Entrepreneurs, The
Crawcour Brothers
History
Tin-mercury dental restorations in China 600
AD
First amalgam War
1980 controversy Huggins, a Practicing
Dentist in Colorado publicly condemned
amalgam
History
Multiple sclerosis, Alzheimers Disease - no
basis
In Japan 1952, mercury was dumped in
Minamata Bay by a local chemical plant
(Chisso Corporation
Physical & chemical properties
ADA Specification No. 6
Mercury exists in 3 chemical forms
Half life of mercury 55 days
Half life of methyl mercury 70 days
http:www.mercury.wikipedia.org.com
Elemental
mercury
Inorganic salts
of mercury
Organic
mercury
Uses of mercury
Elemental mercury:
Sphygmomanometers, thermometers, barometers
Liquid at room temp volatilises easily
Inorganic mercury:
Traditional remedies (ayurvedic, chinese)
Used in gold extraction, caustic soda manufacturing
Rodenticides
Organic mercury:
Fungicides, seed dressings
Methylmercury in fish
Source of mercury
2,700 and 6,000 tons of mercury are released
annually from the oceans and the Earths crust
The greatest source - mercury vapor released
during volcanic eruptions
Uses/Applications
Used in thermometers, barometers, electrical switches, mercury
vapor lamps, fluorescent lamps, paints,
fungicides/insecticides/antiseptics
Dental amalgams, battery manufacturing
2,000 to 3,000 tons are released from human
activities, primarily burning household and
industrial waste
Swordfish and tuna - 1000 g/kg of mass
160 mg/kg in cattle and 25 mg/kg in humans
Methyl mercury compounds are routinely
used as fungicides and herbicides to coat
seeds used to plant farm fields.
Dental fillings are composed of mercury, silver,
tin, copper, and zinc. The approximate
percentages are as follows: 50% mercury, 33-
37% silver, 12.5-13.5% tin 0-3% copper 0-1%
zinc
Mercury is 1 of 2 elements (bromine
is the other)the other) that are liquid at room temperature.
Its elemental symbol is Hg, derived
from the Greek word hydrargyrias,
meaning water silver. This is a fitting
term, since elemental mercury does resemble
liquid silver. The greatest source of
mercury happens to be natural. Outgassing
of granite rock accounts for more than
80% of the mercury found in the atmosphere
and on the earths surface.3 Mercury
is found in many industries, such as battery,
thermometer, and barometer manufacturing.
Some consumer products that
contain mercury include automotive equipment
with halide relay switches, fluorescent
and high-intensity discharge lamps,
and fungicides. Before 1990, paints contained
mercury as an anti-mildew agent. In
medicine, mercury is used in dental amalgams,
as a preservative in vaccines, and in
various antiseptic agents. It
Uses of mercury & its effects
Calomel (mercurous chloride) was used for
the treatment of syphilis.
Inorganic mercury - electrical applications,
chlorine production and dental restorations
Thimerosol as medicine in Hepatitis B,
Diptheria, Pertussis and Tetanus vaccines
Mercury in vaccines have caused death of
infants - diapers
Acrodynia and kawasaki disease teething
powder
Gerald T. Charbenue; Principles and Practices of Operative Dentistry
Biotransformation
Mercury from dental amalgam is released
in two forms
Mercury vapors
Mercuric ions
Inhalation is the major route of entry into the
human body. Metallic mercury is poorly
absorbed to the skin or via the gastro
intestinal tract.
Under normal circumstances, mercury is
biochemically processed and excreted
Mercury ions (Hg
0
) circulate readily in the
blood but pass the membrane barriers of the
brain and placenta with difficulty
Non-ionized mercury (Hg) is capable of
crossing through lipid layers barriers and if
subsequently oxidized within these tissues
neuromuscular problems
Mercury
Mercury was an important constituent of drugs for centuries
as an ingredient in many diuretics, antibacterials, antiseptics,
skin ointments, and laxatives.
More specific, effective, and safer modes of therapy now
have replaced the mercurials, and drug-induced mercury
poisoning has become rare.
However, mercury has a number of important industrial
uses, and poisoning from occupational exposure and
environmental pollution continues to be an area of concern.
Chloralkali (e.g., bleach),Electrical equipment, Paints,
Thermometers, Dental amalgams and Laboratory chemicals.
50
Mercury
Three major chemical forms of the metal must be
distinguished: mercury vapor (elemental mercury), salts
of mercury, and organic mercurials.
Mercury readily forms covalent bonds with sulfur, and it
is this property that accounts for most of the biological
properties of the metal.
Even in low concentrations, mercurials are capable of
inactivating sulfhydryl groups of enzymes and thus
interfering with cellular metabolism and function.
The affinity of mercury for thiols provides the basis for
treatment of mercury poisoning with such agents as
dimercaprol and penicillamine. Mercury also combines
with phosphoryl, carboxyl, amide, and amine groups.
51
Mercury
Elemental mercury is not particularly toxic when
ingested because of very low absorption from the GI
tract. However, inhaled mercury vapor is completely
absorbed by the lung and then is oxidized to the
divalent mercuric cation by catalase in the erythrocytes.
CNS toxicity is more prominent after exposure to
mercury vapor than to divalent forms of the metal.
The soluble inorganic mercuric salts (Hg
2+
) gain access to
the circulation when taken orally. GI absorption is
approximately 10% to 15%. Insoluble inorganic
mercurous compounds, such as calomel (Hg
2
Cl
2
), may
undergo some oxidation to soluble compounds that are
more readily absorbed. Inorganic mercury has a
markedly nonuniform distribution after absorption.
52
Mercury
The highest concentration of Hg
2+
is found in the
kidneys, where the metal is retained longer than in
other tissues.
Inorganic mercurials do not readily pass across the BBB
or the placenta. The metal is excreted in the urine and
feces with a half-life of about 60 days.
More than 90% of Organic mercurials/methylmercury is
absorbed from the human GI tract. The organic
mercurials cross the BBB and the placenta and thus
produce more neurological and teratogenic effects than
do the inorganic salts.
Methylmercury in humans is excreted mainly in the
feces in the form of a glutathione conjugate. The half-
life of methylmercury in the blood of humans is
between 40 and 105 days
53
Mercury vapor toxicity
Short-term exposure: weakness, chills, metallic taste, nausea,
vomiting, diarrhea, dyspnea, cough, and a feeling of tightness in
the chest.
Pulmonary toxicity may progress to an interstitial pneumonitis with
severe compromise of respiratory function.
Recovery, although usually complete, may be complicated by
residual interstitial fibrosis.
Chronic exposure: asthenic (physically weak) vegetative syndrome(
neurasthenic symptoms ,goiter, increased uptake of radioiodine by
the thyroid, tachycardia, labile pulse, gingivitis, dermographia (a
skin condition in which red, itchy lines appear when a person
scratches his or her skin), and increased mercury in the urine.
54
Mercury vapor toxicity
With continued exposure to mercury vapor, tremor
becomes noticeable, and psychological changes consist
of depression, irritability, excessive shyness, insomnia,
reduced self-confidence, emotional instability,
forgetfulness, confusion, impatience, and vasomotor
disturbances (such as excessive perspiration and
uncontrolled blushing, which together are referred to as
erethism = abnormal physical sensitivity).
Common features of intoxication from mercury vapor
are severe salivation and gingivitis.
Renal dysfunction also has been reported to result from
long-term industrial exposure to mercury vapor.
55
Inorganic mercury toxicity
Precipitation of mucous membrane proteins by mercuric
salts (e.g., mercuric chloride) results in an ashen-gray
appearance of the mucosa of the mouth, pharynx, and
intestine and also causes intense pain and vomiting.
The local corrosive effect on the GI mucosa results in
severe hematochezia(passing of fresh blood in a stool)
with evidence of mucosal sloughing in the stool.
Hypovolemic shock and death can occur in the absence
of proper treatment.
Systemic toxicity: a strong metallic taste is followed by
stomatitis (mouth inflammation) with gingival irritation,
foul breath, and loosening of the teeth.
56
Inorganic mercury toxicity
The most serious and frequent systemic effect of
inorganic mercury is renal toxicity(oliguria or anuria).
Renal injury also follows long-term exposure, where
glomerular injury predominates.
The symptom complex of acrodynia also commonly
follows chronic exposure.
Acrodynia: mercury poisoning in young children; mercury
poisoning caused by powders formerly prescribed for
teething, resulting in pinkness and itching of the hands
and feet, excessive sweating, low blood pressure,
limpness, and insomnia
57
Organic Mercurials toxicity
Symptoms of exposure to methylmercury are mainly
neurological and consist of visual disturbance
(scotoma and visual-field constriction), ataxia,
paresthesias, neurasthenia (chronic fatigue), hearing
loss, dysarthria (difficulty in speech articulation),
mental deterioration, muscle tremor, movement
disorders, and with severe exposure, paralysis and
death.
Effects of methylmercury on the fetus can occur even
when the mother is asymptomatic; mental
retardation and neuromuscular deficits have been
observed.
58
Treatment of Mercury Poisoning
Therapeutic measures include immediate termination
of exposure and close monitoring of pulmonary status.
Short-term respiratory support may be necessary.
Inorganic: Prompt attention to fluid and electrolyte
balance and hematological status is of critical
importance in moderate-to-severe oral exposures.
Emesis can be induced if the patient is awake and alert,
although emesis should not be induced where there is
corrosive injury.
59
Treatment of Mercury Poisoning
Chelation therapy with dimercaprol (for high-level
exposures or symptomatic patients) or penicillamine
(for low-level exposures or asymptomatic patients) is
used routinely to treat poisoning with either inorganic
or elemental mercury.
Recommended treatment includes dimercaprol 5 mg/kg
intramuscularly initially, followed by 2.5 mg/kg
intramuscularly every 12 to 24 hours for 10 days.
Penicillamine (250 mg orally every 6 hours) may be used
alone or following treatment with dimercaprol.
The orally effective chelator succimer appears to be an
effective chelator for mercury, although it has not been
approved by the FDA for this purpose.
60
Treatment of Mercury Poisoning
The dimercaprol-mercury chelate is excreted into both
bile and urine, whereas the penicillamine-mercury
chelate is excreted only into urine.
The short-chain organic mercurials, especially
methylmercury, are the most difficult forms of mercury
to mobilize from the body presumably because of their
poor reactivity with chelating agents.
Dimercaprol is contraindicated in methylmercury
poisoning because it increases brain concentrations of
methylmercury in experimental animals.
Methylmercury compounds undergo extensive
enterohepatic recirculation in experimental animals.
61
Treatment of Mercury Poisoning
A nonabsorbable mercury-binding substance into the
intestinal tract should facilitate their removal from the
body.
A polythiol resin has been used for this purpose in
humans and appears to be effective.
The resin has certain advantages over penicillamine. It
does not cause redistribution of mercury in the body
with a subsequent increase in the concentration of
mercury in blood, and it has fewer adverse effects than
do sulfhydryl agents that are absorbed.
Conventional hemodialysis is of little value in the
treatment of methylmercury poisoning because
methylmercury concentrates in erythrocytes, and little is
contained in the plasma.
62
Treatment of Mercury Poisoning
However, it has been shown that L -cysteine can be
infused into the arterial blood entering the dialyzer to
convert methylmercury into a diffusible form.
Both free cysteine and the methylmercury-cysteine
complex form in the blood and then diffuse across the
membrane into the dialysate. This method has been
shown to be effective in humans .
Studies in animals indicate that succimer may be more
effective than cysteine in this regard.
63
Acute toxicity
Chronic toxicity (Hydrargyrism)
Erethism
Neurological
Renal increase plasma creatine level
Immunological
Reproductive
Cardiac
Blood
Liver
Nervous system
Oral manifestations
Thyroid
William. G.Shafer, M.K. Hine; A Text book of Oral Pathology
PINKS disease, Acrodynia, Swift disease - 6 Ps
hands & feet: puffy, pink, painful, peeling,
parasthetia, perspiring
Mercury ions produce toxic effects by
protein precipitation, enzyme inhibition,
and generalized corrosive action. Mercury
not only binds to sulfhydryl groups but
also to phosphoryl, carboxyl, amide, and
amine groups. Proteins (including
enzymes) with such groups readily available
are susceptible to reaction with mercury.
Once bound to mercury, most
proteins are rendered inactive. Toxicity is
in part related to the oxidative state and to
the chemical form (organic versus inorganic).
As mentioned, elemental mercury
vapor is highly lipid soluble which allows
it to readily cross cellular membranes. It
can also be oxidized to the mercuric state.
Since mercuric salts form more soluble
divalent compounds, these forms are more
toxic than the mercurous salts that form
monovalent mercury compounds. Thus,
when ingested they will be more rapidly
absorbed and produce greater toxicity.
Only about 10% of an inorganic salt (regardless
of the oxidative state) is absorbed
compared to 90% absorption via the GI
track of the organic forms. This means the
inorganic forms are available within the GI
track to exert corrosive effects on the gastrointestinal
mucosa.
The organomercurial compounds are
further classified according to chemical
structure and relative toxicity. These
groups are the long-chained arylmercury
compounds and short-chained alkylmercury
compounds. The group that poses the
greater hazard is the short-chained alkyl
compounds, such as methymercury. These
are also most completely absorbed from
the GI tract, distributed to the brain, liver,
and kidney. Excretion is primarily in the
feces. The aryl mercury compounds are
excreted as mercuric ions.
Women who are exposed to mercury in
pregnancy have given birth to children with
serious birth defects due to mercury
poisoning.
In utero mercury exposure can lead to
Minamata disease
Allergy
Others - White cell reaction, reaction to N
2
O,
hyperventilation, reaction to diet pills, gall
bladder, painful menstruation, hepatitis,
severe complexion problems
Treatment
Supportive treatment
DMPS Chelation (2,3-Dimercapto-1-
propanesulphonate)
- Chelation therapy of choice for mercury
- For both acute and chronic mercury
poisoning.
- Urinary excretion of mercury two hours after
DMPS administration
Discontinuance of exposure to mercury .
The administration of BAL (British Anti-
lewisite) pencillamine
Acute mercurialism - Sodium ascorbate
Dr. Olympio Pinto of Rio de Janeiro remove
amalgams when the WBC remained over
11,000 for three months without visible
medical cause
The dental Amalgam Contraversy:A ReviewJCC,1996
N-Acetyl-Cysteine (NAC) For Mercury
Detoxification
NAC is produced in living organisms from
the amino acid cysteine. Thus
NAC is a natural sulfur-containing
amino acid derivative found
naturally in foods and is a powerful
antioxidant
The dental Amalgam Contraversy:A ReviewJCC,1996
Normal levels
Intake of mercury vapor - 0.4 to 4.4 ug/day depending on
number of amalgam fillings.
Mercury concentration in urine - 15 ug/lt
4 nanograms in blood
1 micro gram/ m
3
in air
Tolerable daily intake (TDI) of .014 g Hg
0
/kg-day
(Richardson, 1996)
Richardson, GM; Allan, M. A Monte Carlo assessment of mercury exposure and risks from dental amalgam. Human and
Ecol Risk Assess
Mercury exposure in dental office
Incorrect storage of mercury or waste
amalgam
Spillage of mercury or waste amalgam
Mishandling at any stage would result in
mercury splashing on the bench or floor
causing it to be scattered widely as small
droplets.
Strudevents Art and Science of Operative Dentistry; T.M. Robers
How would you clean up the mercury?
1. Spill on a non-porous surface:
lift the mercury with card or paper (remove gold
rings and wear gloves)
place in a sealed container & dispose in general
waste
2. Spill on a carpet:
Use a sulphur based (calcium polysulfide) powder
mercuric sulphide & then can vacuum up
Strudevents Art and Science of Operative Dentistry; T.M. Robers
Monitoring mercury levels
Detection meter mercury sniffer
Paper discs impregnated with palladium
chloride
Mercury vapour analyzer
A badge system - mercury is adsorbed on gold
foil
Mercury in vapor and dust form - absorbing
system and then quantifying the absorbed
mercury
Mercury monitoring system
Precautions
Mercury-containing products should not be
stored in the open, but rather in closets or
cabinets
Reusable capsules and precapsulated designs -
vacuum aspirator
During trituration
Sprinkling sulfur powder
Scrap amalgam from condensation procedures
Strudevents Art and Science of Operative Dentistry; T.M. Robers
Canadian Dental Association,
1996
Recommendations
Avoid using mercury to restore children's
teeth.
Avoid placing or removing amalgam in the
teeth of pregnant women.
Avoid using dental amalgams in patients
suffering from kidney ailments.
Use methods and equipment to reduce the
risks of exposure to mercury vapor
"The Safety of Dental Amalgam: Health Canada, Dept. of Supply and
Services
Avoid using amalgams in patients who risk suffering
from allergic hypersensitivity.
Remove amalgams from a patient who has become
sensitive.
Avoid placing amalgam in contact with other metal
appliances in the mouth
Fully inform patients of the risks and benefits involved.
Recognize the patient's right to refuse treatment using
a specific material.
The Safety of Dental Amalgam: Health Canada, Dept. of Supply and Services
Mercury hygiene
Minimize the contamination
Drain, vacuum cuspidor or sink is fitted
with a filter, strainer or trap
Spilled mercury - dusting with sulfur
powder or spraying with a solution of
sodium thiosulfate
Mercury spillage kit -->
Strudevents Art and Science of Operative Dentistry; T.M. Robers
Office personnel should also be monitored
Pre-capsulated alloys should be used
Skin contact with mercury should be avoided
Change masks after removing amalgam
restorations
Strudevents Art and Science of Operative Dentistry; T.M. Robers
Mercury contaminated items should be
deposited in sealed bags
Spilled mercury should be cleaned with trap
bottles, taps or fresh mixes of amalgam
The removal of the defective mercury with air
turbine
Strudevents Art and Science of Operative Dentistry; T.M. Robers
Mercury poisoning
Mercury poisoning
- elemental
- inorganic
- organic
Each has a different toxicological profile
There are 3 different forms of mercury
Sources of mercury
Elemental mercury:
Sphygmomanometers, thermometers, barometers
Liquid at room temp volatilises easily
Inorganic mercury:
Traditional remedies (ayurvedic, chinese)
Used in gold extraction, caustic soda manufacturing
Rodenticides
Organic mercury:
Fungicides, seed dressings
Methylmercury in fish
Mercury - Absorption
Inhalation : 60-80%
Dermal : 3-15%
GI Tract : Metallic <0.2%
Inorganic 15%
Organic 90
+
%
Organic mercury poisoning:
Rare but severe
Exposure: ingestion, topical or inhalation
CNS Toxicity:
poor concentration, fatigue, ataxia, tremor, constricted
visual fields,
coma & convulsions
BM suppression
Renal toxicity - dealkylation to inorganic form
Poorer response to treatment
Inorganic mercury poisoning
Gastrointestinal phase: Hg
2+
is a potent GI irritant
gingivitis, stomatitis
- oesophageal, gastric, small and large bowel erosions
- haematemesis, bloody diarrhoea, CVS collapse
Systemic toxicity: Hg
2+
inhibits sulphydryl enzymes
hypotension, lactic acidosis
Nephrotoxicity: Hg
2+
deposits in the tubules ATN
acute renal failure
- potentially leads to CRF in survivors
Elemental Mercury
Case 1: A 4 yr old boy has bitten the top of a
mercury thermometer and his mother thinks he
may have swallowed it.
Little or no risk of toxicity from oral elemental
mercury:
- Faecal excretion precedes slow oxidation
What would your advice be?
Case 2: A man rings A&E because he has
dropped a mercury thermometer in his
sons bedroom.
elemental mercury is volatile
if on a heated surface it may volatalise & be inhaled
once inhaled ~ 80% absorption
What is the risk of toxicity?
Case 2: Mercury thermometer broken in a
bedroom
How would you advise him to clean up the mercury?
1. Spill on a non-porous surface:
lift the mercury with card or paper (remove gold rings and wear
gloves)
place in a sealed container & dispose in general waste
2. Spill on a carpet:
DONT USE a hoover !
Use a sulphur based (calcium polysulfide) powder mercuric
sulphide & then can vacuum up
Large spills: involve environmental health
Inhaled Elemental Mercury (1)
ACUTE
Irritant respiratory effects:
- cough, dyspnoea
- pulmonary oedema, ARDS
Metal fume fever:
- pyrexia, cough, malaise, flu-like symptoms
CNS features:
- confusion, emotional lability, psychoses
- convulsions, CNS depression & coma
Renal effects:
- rarely ARF (oxidation to Hg
2+
)
Erethism
- TREMOR, dysarthria
- peripheral neuropathy, sweating
- personality change
Stomatitis, gingivitis
Chronic renal impairment
Inhaled Elemental Mercury (2)
CHRONIC
Acrodynia
Mercury syndrome in children
Usually related to elemental mercury exposure, 2
reports secondary to inorganic exposure
6 Ps hands & feet: puffy, pink, painful, peeling,
paraesthetic, perspiring
Associated with weight loss, anorexia, irritability,
behavioural changes
Hypertension can mimic phaeochromocytoma
Mercury inhibits COMT (catecholamine-o-
methyltransferase) NAdr / Adr accumulate
Torres AD Pediatrics 2000
IV/IM Elemental Mercury
Results in:
- local complications
- embolic complications
- mercuralism
IV/IM Elemental Mercury
Local Complications
Thrombophlebitis
Infection
Granuloma formation
Excise large s/c deposits
? prevents local & systemic effects
IV/IM Elemental Mercury
Embolic Complications
Pulmonary
- usually asymptomatic
- may cause chest pain, SOB
- normal spirometry, decreased transfer factor
Systemic
- ? mercury through pulmonary capillary bed
- widespread eg. abdomen, intracerebral
- asymptomatic
IV/IM Elemental Mercury
Mercuralism
Slow oxidation of metallic Hg
mercuric ions (Hg
2+
)
Chronic renal impairment
?? CNS toxicity
Consider chelation therapy:
- guided by blood mercury
- may require long-term chelation
Diagnosis of Mercury poisoning
Blood mercury:
only really useful acutely
- normal <10g/l
- symptoms with blood mercury >150-200g/l
Urine mercury
probably the most reliable indicator
- normal <10g/l
- symptoms with urine mercury >100-150g/l
U&E
Radiology: for elemental
ingestion/aspiration/injection
Remove from source
Supportive care
particularly important with inhalation
DMPS Chelation (2,3-Dimercapto-1-
propanesulphonate)
- Chelation therapy of choice for mercury
- For both acute and chronic mercury poisoning
- For all forms of Hg (inorganic > metallic >> organic)
- Indications:
- symptomatic patients
- blood/urine mercury persistently > 100 - 150mg/l
Treatment of Mercury poisoning
Mercury - Hg
Only metal which is liquid at room temperature
Vapours are much more dangerous
Both organic and inorganic compounds, all toxic
Sources: earths crust and industry
Cummulation in water environment
Water microorganisms transform pure mercury into
methylmercury most common source of poisonings
incorporation into food chain (fish)
Other cases of intoxication usually occupational, mistakes
Iraq (wheat seed with antifungal mercury compound
exchanged for food), Minamata disease (fishermen)
Inorganic mercury compounds:
HgCl
2
, Hg(CN)
2
, Hg(NO
3
)
2
Transported bound to blood peptides
Mercury binds with covalent bond to -SH,
-COOH and His
This influences function of many enzymes
and cell processes
Water soluble salts moreover coagulate
peptides and are corrosive
They damage kidney tubules and GIT mucosa
Organic mercury compounds:
Methoxyethylmercury and arylmercury compounds release
mercuric ions act like inorganic compounds
Methyl- and ethylmercury firm bond, whole compound toxic
Bind with Cys and SH, block enzymes, destroy
haematoencephalic (blood-brain) barrier, increase permeability
Don't have corrosive effect on mucosas
In blood transported bound to erythrocytes
They have high affinity to neural tissue
Cummulate also in liver and kidney
Cross the placenta and have fetotoxic effect Excretion to faeces,
urine, milk, sweat, saliva
Deposition in hair and skin, excretion very slow
Clinical signs:
- in dogs, cats and young cattle stimulation of CNS
- in cattle, pigs and poultry depression of CNS
in dogs: blindness, involuntary chewing
in cats: weakness, ataxia, rigidity of hind limbs, convulsions, glass-
like gaze, miaowing, hypersalivation
in calves: ataxia, limping, stumbling, clonuses of eyelids and ears,
hypersensitivity, malfunction of swallowing, disturbance of
vision, convulsions, opistotonus, coma, death
in cattle: anorexia, loss of weight, weakness, loss of coordination,
salivation, lacrimation, diarrhoea and colic, loss of teeth, swollen
lymphatic nodes, disturbances in EKG, cough, dyspnoe, eczema,
hyperkeratosis. Convulsions very rare
in pigs: inappetence, colic, diarrhoea, hypermotility, tremors, loss
of coordination, they are hunched, paralysis of hind limbs
in poultry: ataxia, loss of coordination, pale cere and lobes
Acute intoxication: more often in inorganic poisoning,
mainly GIT signs, oliguria, uraemia, typical mercury bluish
gum line, decrease of blood pressure, CNS disturbances
Chronic intoxication: typical for methylmercury, not GIT
signs, damage of CNS, kidneys, again bluish margin on
gingiva, loss of teeth, tremor
Pathoanatomical examination:
- reduction of cerebellum, leptomeningitis in cats,
congestion and haemorhagia in brain, stomatitis, enteritis,
petechias
- histology swollen axons, demyelinisation, vacuolisation
on neurons, hyperplasia of epithelium
Treatment: chelate agents penicilamin (not dimercaprol
or EDTA!), vitamin E and Se antioxidants, spironolacton
blocks binding of organic mercury compounds to
erythrocytes in acute poisoning
Lead
Through natural occurrence and its industrial use, lead is
ubiquitous(existing every where) in the environment.
The primary sources of environmental exposure to lead
are leaded paint and drinking water; most of the overt
toxicity from lead results from environmental and
industrial exposures.
Occupational exposure to lead has decreased markedly
because of appropriate regulations and programs of
medical surveillance. Workers in lead smelters have the
highest potential for exposure because fumes are
generated, and dust containing lead oxide is deposited in
their environment. Workers in storage-battery factories
face similar risks.
115
Lead
Dietary intake of lead has decreased since the 1940s
due largely to
decrease in the use of lead-soldered cans for food and
beverages;
decrease in the use of lead pipes and lead-soldered joints in
water distribution systems;
introduction of lead-free gasoline;
public awareness of the hazards of indoor leaded paint
The major routes of absorption of lead are from the GIT
and the respiratory system.
Adults absorb approximately 10% of ingested lead,
whereas children absorb up to 40%.
Approximately 90% of inhaled lead particles from
ambient air are absorbed.
116
Lead
After absorption, about 99% of lead in the bloodstream
binds to hemoglobin in erythrocytes. Only 1% to 3% of
the circulating blood lead is in the serum available to
the tissues.
Inorganic lead initially distributes in the soft tissues,
particularly the tubular epithelium of the kidney and in
the liver. In time, lead is redistributed and deposited in
bone, teeth, and hair.
About 95% of the body burden of lead eventually is
found in bone. Only small quantities of inorganic lead
accumulate in the brain, mostly in gray matter and the
basal ganglia.
Iron and Ca
2+
may compete with lead transport.
117
Lead
After establishment of a steady state early in human life,
the daily intake of lead normally approximates the
output, and concentrations of lead in soft tissues are
relatively constant. However, the concentration of lead
in bone apparently increases, and its half-life in bone is
estimated to be 20 to 30 years.
A daily intake of 2.5 mg lead requires nearly 4 years for
the accumulation of a toxic burden, whereas a daily
intake of 3.5 mg requires a few months because
deposition in bone is too slow to protect the soft tissues
during rapid accumulation.
118
Acute Lead Poisoning
Acute lead poisoning is relatively infrequent(acid-soluble
lead compounds or inhalation of lead vapors).
Marked astringency, thirst, and a metallic taste. Nausea,
abdominal pain, and vomiting . Stools may be black from
lead sulfide, and there may be diarrhea or constipation.
If large amounts are absorbed rapidly, a shock syndrome
may develop as the result of massive GI loss of fluid.
Acute CNS symptoms include paresthesias, pain, and
muscle weakness.
An acute hemolytic crisis sometimes causes severe
anemia and hemoglobinuria.
The kidneys are damaged, and oliguria and urinary
changes are evident. Death may occur in 1 or 2 days.
119
Chronic Lead Poisoning
The medical term for lead poisoning is plumbism.
GIT: Anorexia, muscle discomfort, malaise, headache
and Constipation. Severe abdominal pain (lead colic), is
the most distressing feature of the advanced abdominal
syndrome.
In cases where colic is not severe, removal of the patient
from the environment of exposure may be sufficient for
relief of symptoms.
Calcium gluconate administered IV is recommended for
relief of pain and usually is more effective than
morphine
120
Chronic Lead Poisoning
Lead palsy occurs with repeated lead exposure: Muscle
weakness and easy fatigue.
lead encephalopathy, is the most serious manifestation
of lead poisoning and is much more common in
children than in adults.
The early signs of the syndrome include clumsiness,
vertigo, ataxia, falling, headache, insomnia,
restlessness, and irritability. As the encephalopathy
develops, the patient may first become excited and
confused; delirium with repetitive tonic-clonic
convulsions or lethargy and coma follow. Visual
disturbances also are present.
121
Chronic lead toxicity
CDC considers a blood lead concentration of 10 mg/dl
or greater to indicate excessive absorption of lead in
children and to constitute grounds for environmental
assessment, cleanup, and/or intervention.
Chelation therapy should be considered when blood
lead concentrations exceed 25 mg/dl.
Hypochromic microcytic anemia, which is observed
more frequently in children and is morphologically
similar to that resulting from iron deficiency.
Ferrochelatase is the enzyme responsible for
incorporating the ferrous ion into protoporphyrin to
form heme. When ferrochelatase is inhibited by lead,
excess protoporphyrin takes the place of heme in the
hemoglobin molecule.
122
123
Chronic Lead Poisoning
Renal toxicity occurs in two forms: a reversible tubular
disorder (usually seen after acute exposure of children to
lead) and an irreversible interstitial nephropathy
(observed more commonly in long-term industrial lead
exposure).
Ashen color of the face and pallor of the lips; retinal
stippling; appearance of "premature aging," with
stooped posture, poor muscle tone, and emaciation;
and a black, grayish, or blue-black "lead line" along
the gingival margin.
There is a relationship between the concentration of
lead in blood and blood pressure. Lead also interferes
with vitamin D metabolism.
124
Organic Lead toxicity
Tetraethyl lead and tetramethyl lead are lipid-soluble
compounds that are absorbed readily from the skin, GI
tract, and lungs.
The toxicity of tetraethyl lead is believed to be due to
its metabolic conversion to triethyl lead and inorganic
lead.
The major symptoms of intoxication with tetraethyl
lead are referable to the CNS: insomnia, nightmares,
anorexia, nausea and vomiting, diarrhea, headache,
muscular weakness, and emotional instability.
Subjective CNS symptoms such as irritability,
restlessness, and anxiety are next evident, usually
accompanied by hypothermia, bradycardia, and
hypotension.
125
Treatment of Lead Poisoning
Prevention of further exposure is important.
Seizures are treated with diazepam or phenytoin , fluid
and electrolyte balances must be maintained, and
cerebral edema is treated with mannitol and
dexamethasone or controlled hyperventilation.
The concentration of lead in blood should be determined
prior to initiation of chelation therapy.
Chelation therapy is indicated in symptomatic patients or
in patients with a blood lead concentration in excess of
50 to 60 mg/dl.
A chelate is a complex formed between a metal and a
compound that contains two or more potential ligands.
The stability of chelates varies with the metal and the
ligand atoms.
126
Treatment of Lead Poisoning
Four chelators are employed: edetate calcium disodium
(CaNa
2
EDTA), dimercaprol [British antilewisite (BAL)], D-
penicillamine, and succimer [2,3-dimercaptosuccinic
acid (DMSA). CaNa
2
EDTA and dimercaprol usually are
used in combination for lead encephalopathy.
CaNa
2
EDTA is initiated at a dose of 30 to 50 mg/kg per
day in two divided doses either by deep IM injection or
slow IV infusion for up to 5 consecutive days.
Dimercaprol is given IM at a dose of 4 mg/kg every 4
hours for 48 hours, then every 6 hours for 48 hours, and
finally, every 6 to 12 hours for an additional 7 days.
127
Treatment of Lead Poisoning
Penicillamine is effective orally and may be included in
the regimen at a dosage of 250 mg given four times daily
for 5 days. During chronic therapy with penicillamine,
the dose should not exceed 40 mg/kg per day.
Succimer is the first orally active lead chelator available
for children, with a safety and efficacy profile that
surpasses that of D-penicillamine. Succimer usually is
given every 8 hours (10 mg/kg) for 5 days and then every
12 hours for an additional 2 weeks.
In any chelation regimen, the blood lead concentration
should be reassessed 2 weeks after the regimen has
been completed; an additional course of therapy may be
indicated if blood lead concentrations rebound.
128
Chelator
The stability of chelates varies with the metal and the ligand
atoms. For example, lead and mercury have greater affinities
for sulfur and nitrogen than for oxygen ligands; calcium,
however, has a greater affinity for oxygen than for sulfur and
nitrogen. These differences in affinity serve as the basis of
selectivity of action of a chelating agent in the body.
An ideal chelating agent would have the following properties:
high solubility in water, resistance to biotransformation,
ability to reach sites of metal storage, capacity to form
nontoxic complexes with toxic metals, ability to retain
chelating activity at the pH of body fluids, and ready
excretion of the chelate .
129
Chelators
A low affinity for Ca
2+
also is desirable because Ca
2+
in
plasma is readily available for chelation, and a drug
might produce hypocalcemia despite high affinity for
heavy metals.
The most important property of a therapeutic chelating
agent is greater affinity for the metal than that of the
endogenous ligands. The large number of ligands in the
body is a formidable barrier to the effectiveness of a
chelating agent.
130
Arsenic
The foundations of many modern concepts of
chemotherapy derive from Ehrlich's early work with
organic arsenicals, and such drugs once were a
mainstay of chemotherapy.
Arsenic is found in soil, water, and air as a common
environmental toxicant.
The major source of occupational exposure to
arsenic-containing compounds is from the
manufacture of arsenical herbicides and pesticides.
In the manufacture of both computer chips and
semiconductors, metallic arsenic also may be used or
produced as a by-product of the reaction chambers.
131
Arsenic
In general, toxicity increases in the sequence of organic
arsenicals < As
5+
< As
3+
< arsine (AsH
3
).
The organic arsenicals contain arsenic covalently linked
to a carbon atom, where arsenic exists in the trivalent
or pentavalent state. The organic arsenicals usually are
excreted more rapidly than are the inorganic forms.
The pentavalent arsenicals have very low affinity for
thiol groups, in contrast to the trivalent compounds,
and are much less toxic.
Arsine (AsH
3
) is a gaseous hydride of trivalent arsenic; it
produces toxic effects that are distinct from those of
the other arsenic compounds.
132
Arsenic
Arsenate (pentavalent) uncouples mitochondrial
oxidative phosphorylation. The mechanism is thought to
be related to competitive substitution of arsenate for
inorganic phosphate in the formation of ATP.
Trivalent arsenicals, including inorganic arsenite, are
regarded primarily as sulfhydryl reagents. As such,
trivalent arsenicals inhibit many enzymes by reacting
with biological ligands containing available -SH groups.
The arsenite salts are more soluble in water and are
better absorbed than the oxide. Experimental evidence
has shown a high degree of GI absorption (80% to 90%)
of both trivalent and pentavalent forms of arsenic.
133
Arsenic
Arsenic is stored mainly in liver, kidney, heart, and lung.
Much smaller amounts are found in muscle and neural
tissue.
Because of its chemical similarity to phosphorus, it is
deposited in bone and teeth and is retained there for
long periods.
Arsenic readily crosses the placenta, and fetal damage
has been reported.
Arsenic is eliminated by many routes (e.g., feces, urine,
sweat, milk, hair, skin, and lungs), although most is
excreted in urine in humans. The half-life for the urinary
excretion of arsenic is 3 to 5 days, much shorter than
those of the other metals discussed.
134
Toxicity of arsenic
Acute and subacute doses of inorganic arsenic induce
mild vasodilation. Serious CV effects include
hypotension, CHF, and cardiac arrhythmias.
Long-term exposure results in peripheral vascular
disease , more specifically gangrene of the extremities,
especially of the feet, blackfoot disease.
Myocardial damage and hypotension may become
evident after more prolonged exposure to arsenic.
Acute or subacute exposure to arsenic can produce GI
disturbances that range from mild abdominal cramping
and diarrhea to severe hemorrhagic gastroenteritis
associated with shock. With chronic exposure to arsenic,
GI effects usually are not observed.
135
Toxicity of arsenic
Kidney: Initially, the glomeruli are affected, and
proteinuria results. Varying degrees of tubular necrosis
and degeneration occur later. Oliguria with proteinuria,
hematuria, and casts frequently results.
Diffuse or spotted hyperpigmentation over the trunk and
extremities, cutaneous vasodilation and a "milk and
roses" complexion and eventually, skin cancer.
High-dose acute or subacute exposure to arsenic can
cause encephalopathy; however, the most common
arsenic-induced neurological lesion is a peripheral
neuropathy with a stocking/glove distribution of
dysesthesia (constant burning sensation).
This is followed by muscular weakness in the extremities.
136
Toxicity of arsenic
Serious, irreversible blood and bone marrow
disturbances(rare with organic arsenicals).
Fatty infiltration, central necrosis, and cirrhosis.
Skin, bladder ,lung, kidney, and liver cancer.
Chronic exposure to arsenic has been associated with
increased prevalence of diabetes mellitus, goiter,
hepatomegaly, and respiratory system dysfunctions
137
Treatment of Arsenic Poisoning
Routine measures are taken to stabilize the patient and
prevent further absorption of the poison.
Hypotension requires fluid replacement and may
necessitate pharmacological support with pressor
agents such as dopamine.
Chelation therapy often is begun with dimercaprol (3 to
4 mg/kg IM every 4 to 12 hours) until abdominal
symptoms subside and charcoal (if given initially) is
passed in the feces.
Oral treatment with penicillamine then may be
substituted for dimercaprol and continued for 4 days.
Succimer is efficacious in the treatment of arsenic
poisoning.
138
Treatment of Arsenic Poisoning
After long-term exposure to arsenic, treatment with
dimercaprol and penicillamine also may be used, but
oral penicillamine alone usually is sufficient.
Dialysis may become necessary with severe arsenic-
induced nephropathy.
139
Arsenic (As)
Chemistry:
extremely complex because it can exist in metallic form, can be in trivalent and
pentavalent state (charge of 3+ or 5+), and can be organic or inorganic
widely distributed in nature (variety of forms)
Sources:
smelting of gold, silver, copper, lead and zinc ores
combustion of fossil fuels
agricultural uses as herbicides and fungicides
cigarette smoke
occupational: largest source is manufacture of pesticides and herbicides
Environmental fate:
found in surface and groundwater through runoff
accumulates in plants if soil conditions are right
bioaccumulates in aquatic ecosystems (so fish consumption is a source)
From: Klaassen et al., Chap. 19, Philp, Chap. 6
Sources of As
Eating food, drinking water, or breathing air containing arsenic.
Herbal medicines (India/Pakistan Ayurvedic remedies
Breathing contaminated workplace air.
Breathing sawdust or burning smoke from wood treated with arsenic.
Living near uncontrolled hazardous waste sites containing arsenic.
Living in areas with unusually high natural levels of arsenic in rock.
Arsenic: the poison of choice
Napoleon and Paris Green
Arsenic (As)
pharmacokinetics and dynamics:
absorbed via inhalation, ingestion and dermal exposure
mimics phosphate in terms of uptake by cells
Detoxified by methylation: decreased rates lead to
increased toxicity (individual susceptibility)
Can cross placenta
accumulates in liver, kidney, heart and lung - later in
bones, teeth, hair, etc.
half-life is 10 hr, excretion via kidneys
From: Klaassen et al., Chap. 19, Philp, Chap. 6
Arsenic Toxicity Mechanisms
binds to sulfhydryl groups (and disulfide
groups), disrupts sulfhydryl-containing enzymes
(As (III))
inhibits pyruvate and succinate oxidation
pathways and the tricarboxylic acid cycle,
causing impaired gluconeogenesis, and
redu ced oxidative phosphorylation
targets ubiquitous enzyme reactions, so
affects nearly all organ systems
substitution for phosphorus in biochemical
reactions
Replacing the stable phosphorus anion in
phosphate with the less stable As(V) anion
leads to rapid hydrolysis of high-energy
bonds in compounds such as ATP. That leads
to loss of high-energy phosphate bonds and
effectively "uncouples" oxidative
phosphorylation.
Arsenic
Toxicity
organic arsenicals>inorganic
arsenicals>metallic forms
trivalent>pentavalent
acute: severe abdominal pain, fever, cardiac
arrhythmia
chronic: muscle weakness and pain, gross
edema, gastrointestinal disturbances, liver
and kidney damage, swelling of peripheral
nerves (neuritis), paralysis
liver injury: jaundice
peripheral vascular disease - blackfoot
disease
chronic drinking water exposure in
Taiwan and Chile
cancer (skin, lung. Maybe other organs)
Arsenic Toxicity
skin disease:
keratosis of palms and soles, and hyperpigmentation
Medical uses:
Chemotherapy
Causes cancer but also cures it?
Perhaps 2 mechanisms?
Arsenic trioxide approved for acute
promyelocytic leukemia (APL)
Used to be used 100 years ago as
chemo agent, dropped when others
were invented
New interest based on Chinese therapies
70% remission rate
Causes cancer cells to puff up and die
Mechanism: supression of hTERT
gene, which codes for building blocks
of telomerase, which keeps the end
parts of chromosomes intact
Result: chromosomes fuse end-to
end
Case study
Arsenic detection and treatment
What would cause you to suspect As
toxicity?
What tests could you do to detect
exposure or effects?
Mee's lines: white lines on fingernails
can be used to determine chronology
of exposure
What could you prescribe for treatment?
Gastric lavage, activated charcoal
Hemodialysis
BAL chelation
WWII
Arsenic Problems: Bangladesh
As leached from underground sources
into village wells of 1 million people
62% of wells tested exceeded WHO
standard
~ 35 million people exposed above US
EPA standard
200,000 people suffering from As-
induced skin lesions
problem may have been exacerbated
by large scale withdraw of
groundwater for irrigation or by
extensive use of fertilizers
From: Klaassen et al., Chap. 19, Philp, Chap. 6
Arsenic in Drinking Water in the
US
Setting the Standard
1992: California toxicologist
argues that US EPA standard for
As in drinking water would
constitute a 1:100 risk of cancer
for lifetime consumption
EPA standard not originally
based on cancer as an endpoint
achieving a 1:1,000,000 risk
would require dropping
standard from 50 ppb to 2 ppt
EPA revising standard to from
50 to 10 ppb in 2006
consider cost to small
communities
Arsenic On the Playground
Pressure treated wood
CCA: 22 percent pure arsenic
A 12-foot section of pressure-treated
lumber contains about an ounce of
arsenic, or enough to kill 250 people.
"In less than two weeks, an average
five-year-old playing on an arsenic-
treated playset would exceed the
lifetime cancer risk considered
acceptable under federal pesticide
law."
EPA, 2004, banned from residential
use
Poisoned Playgrounds: EWG
Iron
Iron is an essential component of myoglobin; heme
enzymes such as the cytochromes, catalase, and
peroxidase; and the metalloflavoprotein enzymes,
including xanthine oxidase and the mitochondrial
enzyme a-glycerophosphate oxidase.
Iron exists in the environment largely as ferric oxide or
hydroxide or as polymers. In this state, its biological
availability is limited unless solubilized by acid or
chelating agents.
The body store of iron is divided between essential iron-
containing compounds and excess iron, which is held in
storage.
Ferritin is a protein-iron storage complex that exists as
individual molecules or as aggregates.
152
Iron
Internal exchange of iron is accomplished by the
plasma protein transferrin.
Adult men require only 13 mg/kg per day (about 1 mg),
whereas menstruating women require about 21 mg/kg
per day (about 1.4 mg). In the last two trimesters of
pregnancy, requirements increase to about 80 mg/kg
per day (5 to 6 mg), and infants have similar
requirements due to their rapid growth.
Large amounts of ferrous salts are toxic, but fatalities
are rare in adults. Most deaths occur in children,
particularly between the ages of 12 and 24 months. As
little as 1 to 2 g of iron may cause death, but 2 to 10 g
usually is ingested in fatal cases.
153
Iron
Signs and symptoms of severe poisoning may occur
within 30 minutes after ingestion or may be delayed
for several hours.
They include abdominal pain, diarrhea, or vomiting
of brown or bloody stomach contents containing
pills. Of particular concern are pallor or cyanosis,
lassitude (tiredness and apathy), drowsiness,
hyperventilation due to acidosis, and cardiovascular
collapse.
The corrosive injury to the stomach may result in
pyloric stenosis or gastric scarring. Hemorrhagic
gastroenteritis and hepatic damage are prominent
findings at autopsy.
154
Treatment
Emesis
Deferoxamine is poorly absorbed after oral
administration, and parenteral administration is
required in most cases. The drug is administered at
10 to 15 mg/kg per hour by constant infusion. For
chronic iron intoxication , an intramuscular dose of
0.5 to 1.0 g/day is recommended.
An orally effective iron chelator now under clinical
investigation is deferiprone (1,2-dimethyl-3-
hydroxypyridin-4-one),
Shock, dehydration, and acid-base abnormalities
should be treated in the conventional manner.
155
Deferoxamine:deferoxamine mesylate
Isolated as iron chelate from Streptomyces pilosus and is
treated chemically to obtain the metal-free ligand.
Has desirable properties of a remarkably high affinity for
ferric iron coupled with a very low affinity for Ca.
Poorly absorbed after oral administration, and
parenteral administration is required in most cases. For
severe iron toxicity (serum iron levels greater than 500
mg/dl), the IV is preferred. The drug is administered at
10 to 15 mg/kg per hour by constant infusion. Rapid
boluses usually are associated with hypotension.
For chronic iron intoxication (e.g., thalassemia), an
intramuscular dose of 0.5 to 1.0 g/day is recommended.
156
Deferoxamine
Causes a number of allergic reactions, including
pruritus, wheals, rash, and anaphylaxis. Other adverse
effects include dysuria, abdominal discomfort,
diarrhea, fever, leg cramps, and tachycardia.
May cause neurotoxicity during long-term, high-dose
therapy for transfusion-dependent thalassemia major;
both visual and auditory changes have been described.
A "pulmonary syndrome" has been associated with
high-dose (10 to 25 mg/kg per hour) deferoxamine
therapy; tachypnea, hypoxemia, fever, and eosinophilia
are prominent symptoms.
Contraindications: renal insufficiency and anuria;
during pregnancy, the drug should be used only if
clearly indicated.
157
Cadmium
Cadmium (Cd)
Relatively new metal
in terms of humans
Sources:
natural rock
weathering
copper, lead and zinc
smelting auto
exhaust
cigarette smoke (a
cigarette contains 1-
2 ug Cd)
Uses:
metal plating
nickel-cadmium
batteries
solders
paint pigments
(blue)
plastic stabilizers
photographic
chemicals
fungicides
readily absorbed
and accumulated in
plants
Food as most
common route of
exposure for general
population
From: Klaassen et al., Chap. 19, Philp, Chap. 6
Cadmium (Cd)
pharmacokinetics:
inhalation:
smelters, cigarette smoke
15-50% absorbed
ingestion:
main source is liver and kidney of meats
6% absorbed, greater if deficient in calcium,
zinc or iron
Shenyang Copper Smelter
Cadmium (Cd)
pharmacokinetics:
distribution:
bound to albumin in plasma and
red blood cells
transported to liver, pancreas,
prostate and kidney, with eventual
transfer to kidney
50-75% of total body Cd is
found in liver and kidney
Metallothionein: protein rich in cysteine
traps Cd esp. in kidney
synthesis induced by Cd
Elimination: urine
half-life in humans is 20 - 30 years
Metallothionein
Cadmium (Cd)
Toxicity
mechanisms:
binding to SH groups
competing with Zn and Se for inclusion into
metalloenzymes
competing with calcium for binding sites
(calmodulin)
Kidney toxicity:
free Cd binds to kidney glomerulus
proximal tubule dysfunction
From: Klaassen et al., Chap. 19, Philp, Chap. 6
Cadmium (Cd)
Toxicity
Lung toxicity:
edema and emphysema by killing
lung macrophages
Skeletal effects:
Osteoporosis and osteomalacia
(pseudofractures)
Cancer:
carcinogenic in animal studies
~8% of lung cancers may be
attributable to Cd
From: Klaassen et al., Chap. 19, Philp, Chap. 6
Cadmium detection and
treatment
What would cause you to suspect Cd toxicity?
What tests could you do to detect exposure or
effects?
detected via increased excretion of
proteins, amino acids and calcium
What could you prescribe for treatment?
Acute inhalation: fluid replacement,
mechanical ventilation
Acute ingestion: emesis and gastric lavage
Chronic:
chelation therapy is ineffective so only
treatment is to remove source
Cadmium (Cd)
Epidemics/case studies
Japan (1940s)
effluent (outflow) from a lead-
processing plant washed over adjacent
rice paddies for many years
rice accumulated high level of Cd
community was poor (and
therefore malnourished with
respect to calcium)
acute toxicity: renal
failure,anemia, severe muscle pain
named "Itai-Itai" disease
("ouch, ouch")
From: Klaassen et al., Chap. 19, Philp, Chap. 6
Itai-itai victim
Cadmium (Cd)
Epidemics/case studies
Sewage waste disposal
contains high levels of N and P, and
organic matter, so makes sense to
use as fertilizer on fields
also can contain Cd, which is
readily accumulated in plants
livestock grazing on fields can
accumulate Cd in liver and kidney
6-8x as much as sheep grazing
on clean fields
From: Klaassen et al., Chap. 19, Philp, Chap. 6
Land application
Cadmium - Cd
No constructive purpose in the body
Extremely toxic even in low concentrations,
accumulates in organisms and ecosystems
Chemical properties similar to zinc exchange in an
organism (also can replace Cu, Fe, Ca)
Sources: earth crust, fossil fuels, plastic materials
industry, electronic industry, tobacco fume
Absorption after digestion or less by inhalation (but
here better bioavailability)
The first documented case of mass cadmium poisoning
in the world - in Toyama Prefecture, Japan in 1950
Itai-Itai disease (river polluted with waste from factory,
water used on rice fields poisoning from rice)
- In blood transported bound to proteins (formation
of complexes), in higher concentrations bound to
erythrocytes
- Deposition in liver, kidneys and gonads
Mechanism of action:
- Inhibition of many enzymes, antagonist to many
metals Zn, Cu, Ca, Fe
- Disturbance of cholecalciferol production, thus
influences Ca metabolism
- Inhibition of specific hydrolase in testis affects
activity of gonads
- Formation of complexes, which are digested in
kidney release of Cd damage
- Xenoestrogennic effect
Clinical signs:
- Acute exposure:
- Cadmium fumes may cause flu like symptoms
including chills, fever, and muscle ache
- More severe exposures can cause tracheo-
bronchitis, pneumonitis, and pulmonary oedema.
Symptoms of inflammation may start hours after
the exposure and include cough, dryness and
irritation of the nose and throat, headache,
dizziness, weakness, fever, chills, and chest pain.
- Ingestion of any significant amount of cadmium
causes immediate poisoning and damage to the
liver and the kidneys. Also CNS disturbances occur
and changes in blood count
- Chronic exposure:
- Osteomalacia, osteoporosis disturbance of vitamin
D and calcium metabolism
- Pain in the joints and the back, and also increases
the risk of fractures. In extreme cases of cadmium
poisoning, the mere body weight causes a fracture
- The kidneys lose their function to remove acids
from the blood. The kidney damage inflicted by
cadmium poisoning is irreversible and does not heal
over time.
- Gout, a form of arthritis due to the accumulation of
uric acid crystals in the joints (hyperuricemia).
- Some patients may lose their sense of smell
(anosmia)
- Damage of gonads, suspected carcinogen tumours
of testes
Pathological examination:
- gastritis, enteritis, nephritis, stomatitis,
degeneration of liver, necrosis on testes
Treatment :
- Chelating agents EDTA only partially effective
- Administration of calcium
Вам также может понравиться
- Radiation Protective Foods: How to Shield Yourself from Low-Level RadiationОт EverandRadiation Protective Foods: How to Shield Yourself from Low-Level RadiationОценок пока нет
- Heavy Metals Acting As Endocrine DisruptersДокумент5 страницHeavy Metals Acting As Endocrine Disrupters300rОценок пока нет
- Veterinary ToxicologyДокумент822 страницыVeterinary ToxicologyPau Ibarra RieraОценок пока нет
- (Recent Trends in Biotechnology) Johanna Brewer-Forensic Science - New Developments, Perspectives and Advanced Technologies-Nova Science Pub Inc (2015) PDFДокумент141 страница(Recent Trends in Biotechnology) Johanna Brewer-Forensic Science - New Developments, Perspectives and Advanced Technologies-Nova Science Pub Inc (2015) PDFreflisampeОценок пока нет
- Oxidation of Amino Acids, Peptides, and Proteins: Kinetics and MechanismОт EverandOxidation of Amino Acids, Peptides, and Proteins: Kinetics and MechanismОценок пока нет
- Vpt421 Veterinary Toxicology Tanuvas Lecture NotesДокумент123 страницыVpt421 Veterinary Toxicology Tanuvas Lecture NotesMohd ZubairОценок пока нет
- Role of Molecular Chaperones on Structural Folding, Biological Functions, and Drug Interactions of Client ProteinsОт EverandRole of Molecular Chaperones on Structural Folding, Biological Functions, and Drug Interactions of Client ProteinsРейтинг: 2 из 5 звезд2/5 (1)
- Instrumental Methods For The Analysis and Identification of Bioactive MoleculesДокумент370 страницInstrumental Methods For The Analysis and Identification of Bioactive MoleculesSuyog patilОценок пока нет
- Dermatological and Haemato-Biochemical Characteristics of Canine HypothyroidismДокумент5 страницDermatological and Haemato-Biochemical Characteristics of Canine HypothyroidismsumОценок пока нет
- Ocular Issues and Endocrine Diseases in Small Animal Practice (Canine Eye Disease)Документ11 страницOcular Issues and Endocrine Diseases in Small Animal Practice (Canine Eye Disease)maddierogersОценок пока нет
- Analytical Toxicology PDFДокумент62 страницыAnalytical Toxicology PDFPraditya AgungОценок пока нет
- Toxicology Laboratory ManualДокумент110 страницToxicology Laboratory Manualnguyen ba trung100% (1)
- MSC Medical PhysicsДокумент39 страницMSC Medical PhysicsHimanshi ParkarОценок пока нет
- Forensic ToxicologyДокумент11 страницForensic ToxicologySeptiandry Ade PutraОценок пока нет
- Toxicological Stability of Drugs: de Jesus, Adia Cavrinni G. Manalo, Alyssa AДокумент42 страницыToxicological Stability of Drugs: de Jesus, Adia Cavrinni G. Manalo, Alyssa AAlyssa ManaloОценок пока нет
- Lecture 2 Population GeneticsДокумент16 страницLecture 2 Population Geneticsimorkzone0% (1)
- ANTI INFLAMMATORY Screening MethodsДокумент7 страницANTI INFLAMMATORY Screening MethodsBrajesh Thankamony67% (3)
- The Biological Effect of NanoparticlesДокумент16 страницThe Biological Effect of NanoparticlesmihaelaputinaОценок пока нет
- Radio Pharmaceuticals First LectureДокумент41 страницаRadio Pharmaceuticals First LectureHagar MoslehОценок пока нет
- Prepared By: Shella Mae N. Mainit MSF-1Документ44 страницыPrepared By: Shella Mae N. Mainit MSF-1Chriseil arts tancioОценок пока нет
- Laboratory SafetyДокумент4 страницыLaboratory SafetyLey DoydoraОценок пока нет
- S Under The MicroscopeДокумент41 страницаS Under The MicroscopeBruno SarmentoОценок пока нет
- Nanopharmaceuticals-iEvaluation Guidelines DraftДокумент49 страницNanopharmaceuticals-iEvaluation Guidelines DraftCK KatiyarОценок пока нет
- Radiation BiologyДокумент155 страницRadiation BiologyAliHasanCanoğluОценок пока нет
- Ethnoveterinary Practice Promoting Indigenous KnowledgeДокумент33 страницыEthnoveterinary Practice Promoting Indigenous KnowledgePratap Kafle100% (2)
- G.N. Ramachandran: Published: 01 June 2001Документ7 страницG.N. Ramachandran: Published: 01 June 2001Naina MarbusОценок пока нет
- Photobiology PPT by DR AkbarДокумент27 страницPhotobiology PPT by DR AkbarAyesha awanОценок пока нет
- Veterinary Infection Biology Molecular Diagnostics and High-Throughput Strategies (VetBooks - Ir)Документ557 страницVeterinary Infection Biology Molecular Diagnostics and High-Throughput Strategies (VetBooks - Ir)MAGDA YOANA BELTRAN LEON100% (1)
- Top 7 - Indian's Favourite Dog Breed in 2020Документ9 страницTop 7 - Indian's Favourite Dog Breed in 2020PawrulzОценок пока нет
- Top 10 Dog Breeds in IndiaДокумент13 страницTop 10 Dog Breeds in IndiaSatish KaushikОценок пока нет
- Toxicological StudiesДокумент47 страницToxicological StudiesMadhu ShaliniОценок пока нет
- Veterinary Forensic ToxicologyДокумент11 страницVeterinary Forensic ToxicologyCarlos Alberto Chaves VelasquezОценок пока нет
- 0000 - An Introduction To ToxicologyДокумент21 страница0000 - An Introduction To ToxicologyStella SamkyОценок пока нет
- Plasmid Profiling of Crude Petroleum Degrading Bacterial Strains Isolated From Polluted Soils in Ota, NigeriaДокумент11 страницPlasmid Profiling of Crude Petroleum Degrading Bacterial Strains Isolated From Polluted Soils in Ota, NigeriaObafemi YemisiОценок пока нет
- Metal Poisoning - Mercury, Lead, Cadmium: Lecture No. 9Документ19 страницMetal Poisoning - Mercury, Lead, Cadmium: Lecture No. 9lauraОценок пока нет
- Lecture10 Cu, ZN PoisoningДокумент21 страницаLecture10 Cu, ZN PoisoninglauraОценок пока нет
- Heavy Metals: Dela Cruz, Maricar Joyce Malacad, Josefina Angela Villanueva, JamaykaДокумент52 страницыHeavy Metals: Dela Cruz, Maricar Joyce Malacad, Josefina Angela Villanueva, JamaykaCristina SarinoОценок пока нет
- Metals (PB, HG, MN, CD, As, Ni, CR)Документ49 страницMetals (PB, HG, MN, CD, As, Ni, CR)Йеша Маниш МираниОценок пока нет
- 16 Heavy MetalsДокумент42 страницы16 Heavy MetalsHeba AlhasiОценок пока нет
- Toxicology (6, 7)Документ44 страницыToxicology (6, 7)medov43233Оценок пока нет
- Heavy Metals and Their AntagonistsДокумент42 страницыHeavy Metals and Their AntagonistsMohammad AzharuddinОценок пока нет
- Mineral PoisoningДокумент35 страницMineral PoisoningFira'ol BogalaОценок пока нет
- MineralsДокумент26 страницMineralsامجد حسين جواد كاظمОценок пока нет
- Heavy Metals and Heavy Metal Antagonist Final OutputДокумент124 страницыHeavy Metals and Heavy Metal Antagonist Final OutputAdityaОценок пока нет
- Carbon Monoxide PoisoningДокумент22 страницыCarbon Monoxide PoisoningSunilОценок пока нет
- علم السمومДокумент7 страницعلم السمومBouna BounaОценок пока нет
- #Heavy MetalsДокумент2 страницы#Heavy MetalsameerabestОценок пока нет
- Lead PoisoningДокумент17 страницLead PoisoningParvez X GulОценок пока нет
- Unit VII Environmental Pollution and Heavy Metal Poisons BPHДокумент29 страницUnit VII Environmental Pollution and Heavy Metal Poisons BPHGanesh PoudelОценок пока нет
- Nutritional Toxic Causes of Cardiomyopathy:: ToxinsДокумент3 страницыNutritional Toxic Causes of Cardiomyopathy:: Toxinsebh0726Оценок пока нет
- Electrolytes and Its FunctionДокумент28 страницElectrolytes and Its Functionfirgpunk91Оценок пока нет
- MineralsДокумент32 страницыMineralsMuhammad ZohaibОценок пока нет
- MineralsДокумент38 страницMineralslovi bahunОценок пока нет
- 19.20heavy MetalsДокумент19 страниц19.20heavy MetalsallemedizinОценок пока нет
- Lecture 33 - Vitamins & Trace ElementsДокумент35 страницLecture 33 - Vitamins & Trace Elementsapi-3703352100% (1)
- Boards ReviewДокумент129 страницBoards ReviewJoanneYiОценок пока нет
- Water, Electrolyte, & Acid-Based Balance: Powerpoint Designed by Fellow Student J.HДокумент14 страницWater, Electrolyte, & Acid-Based Balance: Powerpoint Designed by Fellow Student J.HTaranatorОценок пока нет
- Chapter3 - Chemical AgentsДокумент64 страницыChapter3 - Chemical AgentsadillaaazmiОценок пока нет
- Endocrine Vety PharmaДокумент16 страницEndocrine Vety PharmaSunilОценок пока нет
- Mycotoxins in Animal HealthДокумент103 страницыMycotoxins in Animal HealthSunil100% (1)
- Household Hazards To PetsДокумент16 страницHousehold Hazards To PetsSunilОценок пока нет
- Carbon Monoxide PoisoningДокумент22 страницыCarbon Monoxide PoisoningSunilОценок пока нет
- Fluorosis Phosphorous Nitrate Toxicity in AnimalsДокумент55 страницFluorosis Phosphorous Nitrate Toxicity in AnimalsSunilОценок пока нет
- VPT MCQ ForДокумент10 страницVPT MCQ ForSunilОценок пока нет
- Classification of AmphibiansДокумент22 страницыClassification of AmphibiansSunilОценок пока нет
- Nsaids and Other Antinflammatory Agents in Veterinary PracticeДокумент44 страницыNsaids and Other Antinflammatory Agents in Veterinary PracticeSunil100% (2)
- Toxicokinetics DynamicsДокумент76 страницToxicokinetics DynamicsSunil100% (1)
- Drugs in ReptilesДокумент71 страницаDrugs in ReptilesSunil100% (1)
- Drugs in Behavioural Disorders of PetsДокумент5 страницDrugs in Behavioural Disorders of PetsSunilОценок пока нет
- Antiseptics and Disinfectants For Veterinary ClinicsДокумент3 страницыAntiseptics and Disinfectants For Veterinary ClinicsSunil100% (5)
- Toxicological Investigation and Its Significance in Animal Health DiagnosisДокумент8 страницToxicological Investigation and Its Significance in Animal Health DiagnosisSunilОценок пока нет
- Classification and Dosage of Antimicrobial Agents in Veterinary MedicineДокумент24 страницыClassification and Dosage of Antimicrobial Agents in Veterinary MedicineSunil0% (1)
- VCI MSVE 2008 RegulationsДокумент136 страницVCI MSVE 2008 RegulationsSunil50% (2)
- Drugs Acting On Genitourinary System of AnimalsДокумент44 страницыDrugs Acting On Genitourinary System of AnimalsSunilОценок пока нет
- Drugs Acting On Haematopoietic System of AnimalsДокумент28 страницDrugs Acting On Haematopoietic System of AnimalsSunil100% (1)
- Behavioural Modifying Drugs in PetsДокумент33 страницыBehavioural Modifying Drugs in PetsSunilОценок пока нет
- Drugs Acting On Respiratory System of AnimalsДокумент8 страницDrugs Acting On Respiratory System of AnimalsSunil100% (4)
- LATHYRISM AND PHOTOSENSiTIZATIONДокумент33 страницыLATHYRISM AND PHOTOSENSiTIZATIONSunilОценок пока нет
- GENERAL LINE of Treatment UREA AMMONIA SALT - poISONINGДокумент49 страницGENERAL LINE of Treatment UREA AMMONIA SALT - poISONINGSunil0% (1)
- Drugs Acting On Digestive System of AnimalsДокумент11 страницDrugs Acting On Digestive System of AnimalsSunil100% (3)
- ZOOTOXINSДокумент72 страницыZOOTOXINSSunil83% (6)
- Mycotoxins in Animal HealthДокумент103 страницыMycotoxins in Animal HealthSunil100% (1)
- Site of Action of Drugs Acting On Adrenergic Neurohumoral Transmission PDFДокумент1 страницаSite of Action of Drugs Acting On Adrenergic Neurohumoral Transmission PDFSunilОценок пока нет
- Site of Action of Drugs Acting On Cholinergic Neurohumoral Transmission PDFДокумент1 страницаSite of Action of Drugs Acting On Cholinergic Neurohumoral Transmission PDFSunilОценок пока нет
- Fluorosis Phosphorous ToxicityДокумент55 страницFluorosis Phosphorous ToxicitySunilОценок пока нет
- Environmental PollutantsДокумент32 страницыEnvironmental PollutantsSunilОценок пока нет
- AntiConvulsants Drugs in Brief PDFДокумент28 страницAntiConvulsants Drugs in Brief PDFSunilОценок пока нет
- Unn Medical SciencesДокумент133 страницыUnn Medical SciencesNNANEMERE FAVOUR CHIDIMMAОценок пока нет
- Variation of Soil Microbial Population in Different Soil HorizonsДокумент4 страницыVariation of Soil Microbial Population in Different Soil HorizonsprabhatОценок пока нет
- Pricelist Juli 2023 UserДокумент108 страницPricelist Juli 2023 Userfitri heriyati pratiwiОценок пока нет
- Chapter 6. Fabric FiltersДокумент51 страницаChapter 6. Fabric Filterspekanselandar100% (1)
- Obturating Materials Used For Pulpectomy in Primary Teeth A ReviewДокумент9 страницObturating Materials Used For Pulpectomy in Primary Teeth A ReviewMohammed SaeedОценок пока нет
- Mark Scheme (Results) : Summer 2018Документ17 страницMark Scheme (Results) : Summer 2018Atiqur RahmanОценок пока нет
- Chem Final Notes UsydДокумент5 страницChem Final Notes UsydRobs0% (1)
- Design, Installation and Fabrication of Reciprocating PumpДокумент40 страницDesign, Installation and Fabrication of Reciprocating PumpMehdi Baghaie78% (9)
- Chemical MachiningДокумент5 страницChemical MachiningomenОценок пока нет
- Soil PH and Soil Acidity PDFДокумент16 страницSoil PH and Soil Acidity PDFManuel EscobarОценок пока нет
- Primacs TOC Analyser: Chapter 1: IntroductionДокумент12 страницPrimacs TOC Analyser: Chapter 1: IntroductionAnonymous 2LYCWDPuiuОценок пока нет
- 120131-Perforated Sheet Metal - IPRF - CD PDFДокумент239 страниц120131-Perforated Sheet Metal - IPRF - CD PDFMisagh100% (1)
- Yuanping Wang Chuqin Yu: Preparation and in Vitro Dissolution of Curcumin Tablets,, Zhixiang Gan, Zhongbo XieДокумент7 страницYuanping Wang Chuqin Yu: Preparation and in Vitro Dissolution of Curcumin Tablets,, Zhixiang Gan, Zhongbo XieChristineОценок пока нет
- Prime, Tack & Fog 2019 SL PDFДокумент42 страницыPrime, Tack & Fog 2019 SL PDFLucia SaezОценок пока нет
- Exp 10 Alkyl HalidesДокумент18 страницExp 10 Alkyl HalidesGeorge PiliposyanОценок пока нет
- ISO14001 Step by Step Guide APMM VesselsДокумент36 страницISO14001 Step by Step Guide APMM Vesselsnasol100% (3)
- ReviewerДокумент19 страницReviewerCarlos Miguel Dacaimat100% (1)
- Mixed-Use Development One Binondo: Moriones St. Cor. Juan Luna St. Binondo, ManilaДокумент1 страницаMixed-Use Development One Binondo: Moriones St. Cor. Juan Luna St. Binondo, ManilaedwinОценок пока нет
- Chemistry of Kupipakwa RasayanasДокумент7 страницChemistry of Kupipakwa RasayanasSrinivas Naik0% (1)
- Design of Concrete Buildings For Disassembly: An Explorative ReviewДокумент19 страницDesign of Concrete Buildings For Disassembly: An Explorative Reviewmonica singhОценок пока нет
- AMPCO 18 SandДокумент1 страницаAMPCO 18 SandS BHATTACHARYYAОценок пока нет
- TS TVS TFS ProtocolsДокумент16 страницTS TVS TFS ProtocolsTaulehia Pulefou SemisiОценок пока нет
- Recycling Engine CoolantДокумент9 страницRecycling Engine CoolantYendi Kesuma100% (1)
- Eu3c6 by Adel KhamisДокумент31 страницаEu3c6 by Adel KhamisAdel KhamisОценок пока нет
- Synthesis and Characterization of Cerium Oxide Nanoparticles Using Different Solvents For Electrochemical ApplicationsДокумент10 страницSynthesis and Characterization of Cerium Oxide Nanoparticles Using Different Solvents For Electrochemical ApplicationsVengateshwaran TDОценок пока нет
- Engineering MaterialsДокумент16 страницEngineering MaterialsYosef Ganang Jati NugrohoОценок пока нет
- Failure of 9FA Gas Turbine Compressor - A Unique Experience. D.Nandi (AGM-OS/GT) K.R.C.Murty (AGM-OS/GT)Документ10 страницFailure of 9FA Gas Turbine Compressor - A Unique Experience. D.Nandi (AGM-OS/GT) K.R.C.Murty (AGM-OS/GT)Thanapaet RittirutОценок пока нет
- Paper - I - General: Previous Year Paper GPAT DIДокумент20 страницPaper - I - General: Previous Year Paper GPAT DIVikash KushwahaОценок пока нет
- ECV5701 Notes For Concrete Inspection and Assessment-1Документ19 страницECV5701 Notes For Concrete Inspection and Assessment-1ahmed almhjani100% (1)
- Basics of SMAWДокумент157 страницBasics of SMAWAsad Bin Ala QatariОценок пока нет