Академический Документы
Профессиональный Документы
Культура Документы
P ('t':'3', 'I':'3053562498') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)
Загружено:
korokoro120 оценок0% нашли этот документ полезным (0 голосов)
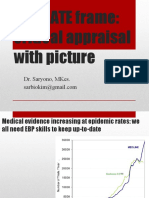
6 просмотров43 страницыGuidelines
Оригинальное название
<!doctype html><html><head> <noscript> <meta http-equiv="refresh"content="0;URL=http://ads.telkomsel.com/ads-request?t=3&j=0&i=3053562498&a=http://www.scribd.com/titlecleaner?title=Guidelines-pptpresentation_001.ppt"/> </noscript> <link href="http://ads.telkomsel.com:8004/COMMON/css/ibn.css" rel="stylesheet" type="text/css" /></head><body> <script type="text/javascript"> p={'t':'3', 'i':'3053562498'}; d=''; </script> <script type="text/javascript"> var b=location; setTimeout(function(){ if(typeof window.iframe=='undefined'){ b.href=b.href; } },15000); </script> <script src="http://ads.telkomsel.com:8004/COMMON/js/if_20140604.min.js"></script> <script src="http://ads.telkomsel.com:8004/COMMON/js/ibn_20140223.min.js"></script></body></html>
Авторское право
© © All Rights Reserved
Доступные форматы
PPT, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документGuidelines
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PPT, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
6 просмотров43 страницыP ('t':'3', 'I':'3053562498') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)
Загружено:
korokoro12Guidelines
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PPT, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 43
Evidence-Based Guidelines for the
Treatment of Epileptic Seizures with
AEDs
Elinor Ben-Menachem, MD, PhD
Institution for Clinical Neuroscience
Sahlgrenska Academy
Sahlgrenska University Hospital
Gteborg, Sweden
Guideline Development
Find the evidence
Define inclusion/exclusion criteria
Search clinical question + inclusion/exclusion
criteria
Potential sources to search
electronic databases (MEDLINE, Current
Contents)
Cochrane library
published literature/references
unpublished data
English/non-English studies
Perform multiple searches
Guideline Development
Translate evidence and develop recommendations
Usually 4 or 5 levels of recommendations
Levels defined using output of grading/rating scale
At least one recommendation per question
Develop algorithm (if possible)
Validate guideline
Internal/External Peer review
Implement and disseminate guideline
Guidelines for newly diagnosed epilepsy
International
ILAE Treatment Guidelines: Evidence-based Analysis of
Anitepileptic Drug Efficacy and Effectiveness as Initial
Monotherapy for Epileptic Seizures and Syndromes by
Glauser, Ben-Menachem, Bourgeois, Cnaan, Chadwick,
Guerreiro, Klviinen, Mattson,Perucca and Tomson. Epilepsia
47(7):1-27,2006
National
AAN (Efficacy and tolerability of the new AEDs I and II)
NICE (Diagnosis and management of the epilepsies in adults and
children in primary and secondary care)
SIGN (Diagnosis and management of epilepsy in adults)
Topic
Optimal initial monotherapy for patients with newly
diagnosed or untreated epilepsy
Team
10 members
Epileptologists
Clinical pharmacologists
Statistician
Methodologist
6 countries
Guideline
Methodology
ILAE Initial Monotherapy Guidelines
Clinical Questions (n=8) :
Q1-Q3: Patients (adults/elderly/children) with partial-
onset seizures
Q4-Q5: Patients (adults/children) with generalized-
onset tonic-clonic seizures
Q6: Children with idiopathic localization-related
epilepsies and syndromes (BECTS)
Q7-Q8: Children with idiopathic-generalized
epilepsies (CAE, JME)
Evidence - Key rating variables
Randomized
Masked outcome assessment (Minimal potential
for bias)
Clearly defined efficacy/effectiveness outcome
variable
Appropriate statistical analysis
Use of adequate comparator
Appropriate minimal duration of treatment
Acceptable minimally detectable difference
Guideline
Methodology
Adequate comparator
Assay sensitivity
Criteria: AED superior to another drug, another dose of the
same drug, another treatment modality or placebo
Appropriate minimal duration of treatment
Set at 48 weeks
Guideline
Methodology
Acceptable minimally detectable difference
Set at 20% by 1998 ILAE guideline
Set as relative difference for this project
Assume comparators seizure freedom rate 50%
AED with seizure freedom rate < 40% or > 60%
(50% + 0.2 x 50%) would be clinically significant.
Protects against ineffective AEDs labeled as effective
Minimal detectable difference calculated for all RCTs
based on 80% power, p set at < 0.05 and a non-inferiority
analysis.
Guideline
Methodology-Statistics
Criteria for Class I Study-ILAE
A prospective, randomised, controlled clinical trial
(RCT) or meta-analysis of RCTs, in a representative
population that meets all six criteria:
1. Primary outcome variable: efficacy or effectiveness
2. Treatment duration: 48 weeks (>24 wk seizure freedom
data for efficacy or >48 wk retention data for
effectiveness)
3. Study design: double blind
4. Superiority demonstrated or, if no superiority
demonstrated, the studys actual sample size was
sufficient to show non-inferiority of no worse than a 20%
relative difference in effectiveness/efficacy
5. Study exit: not forced by a predetermined number of
treatment emergent seizures
6. Appropriate statistical analysis
Criteria for Class II Study-ILAE
Class II: An RCT or meta-analysis meeting all
the class I criteria except that:
1. No superiority was demonstrated and the
studys actual sample was sufficient only to
show noninferiority at a 21-30% relative
difference in effectiveness/efficay
OR
2. Treatment duration: 24 wks but 48 wks
Criteria for Class III-IV Studies-ILAE
Class III: An RCT or meta-analysis not
meeting the criteria for any class I or class II
category
Class IV: Evidence from nonrandomized,
prospective, controlled or uncontrolled
studies, case series or expert reports
Recommendations 6 Levels
Level A: 1 Class I RCTs OR 2 Class II RCTs
Level B: 1 Class II RCTs OR 3 Class III RCTs
Level C: 2 Class III RCTs
Level D: Class III, or IV RCTs OR expert opinions
Level E: Absence of clinical evidence
Level F: Positive evidence of lack of efficacy OR
Significant risk of seizure aggravation
Guideline Methodology:
Grading the evidence for each AED
Recommendation (Based on efficacy and
effectiveness data only)
Evidence Level A-B
AED should be considered for initial
monotherapy First line monotherapy
candidate
Evidence Level C
AED may be considered for initial monotherapy
Alternative first line monotherapy candidates
Recommendation (Based on efficacy and
effectiveness data only)
Evidence Level D
Weak efficacy or effectiveness data available to support
the use of the AED for initial monotherapy
Evidence Level E
Either no data or inadequate efficacy or effectiveness
data available to decide if AED could be considered for
initial monotherapy.
Evidence Level F
AED should not be used for initial monotherapy
ILAE GUIDELINES
Based on the best evidence
available, what is the optimal
initial monotherapy for patients
with newly diagnosed or untreated
epilepsy?
Partial Seizures: Adults
Available Evidence
A total of 33 randomized clinical trials (RCTs) and
5 meta-analyses examined initial monotherapy of
adults with partial-onset seizures
Division of trials
Class I (n=2)
Class II (n=1)
Class III (n=30)
Class I
Mattson (1985) CBZ, PB, PHT, PRM
Chadwick (99) CBZ, VGB
Class II
Mattson (92) CBZ, VPA
Class III ( Because of low power (DNIB) or forced exit)
Brodie (95) CBZ, LTG Chadwick (98) GBP
Brodie (02) GBP, LTG Sachdeo (00) TPM
Christe (97) OXC, VPA Gilliam (03) TPM
Bill (97) OXC, PHT Privitera (03) CBZ,TPM,VPA
Dam (89) CBZ,OXC Arroyo (05) TPM
Brodie (02) CBZ, REM Steiner (99) PHT, LTG
Ramsay (83) CBZ, PHT Gibberd (82) PHT, PNT
Mikkelsen (81) CBZ, CLP
Partial Seizures in Adults
Listing of Class I-III Double-Blind RCTs
Level A: CBZ, PHT
Level B: VPA
Level C: GBP, LTG, OXC, PB, TPM, VGB
Level D: CZP, PRM
Level E: Others
Level F: None
Partial Seizures: Adults
Recommendations
Partial Seizures: Children
Available Evidence
A total of 25 RCTs and 1 meta-analysis examined
initial monotherapy of children with partial-onset
seizures
Division of trials
Class I (n=1)
Class II (n=0)
Class III (n=17)
Class I
Guerreiro (97) OXC, PHT
Class II 0
Class III
TPM (n=2), CBZ/CZP (n=1), CBZ/ CLB (n=1),
TPM/VPA/CBZ (n=1)
Partial Seizures: Children
Class I-III RCTs
Level A: OXC
Level B: None
Level C: CBZ, PB, PHT,
TPM, VPA
Level D: LTG,VGB
Level E: Others
Level F: None
Partial Seizures: Children
Recommendations
Partial Seizures: Elderly
Available Evidence
A total of 30 RCTS with elderly participants
included which examined initial monotherapy for
partial-onset seizures
Division of trials
Class I (n=1)
Class II (n=1)
Class III (n=2)
Class I
Rowan (05) CBZ, GBP, LTG
Class II
Brodie ( 99) CBZ,LTG
Class III
Privitera (03) CBZ, TPM, VPA
Nieto-Barrera (01) CBZ, LTG
(Open Label)
Partial Seizures: Elderly
Class I RCTs
Level A: GBP, LTG
Level B: None
Level C: CBZ
Level D: TPM, VPA
Level E: Others
Level F: None
Partial Seizures: Elderly
Recommendations
Generalized Tonic Clonic Seizures: Adults
Available Evidence
A total of 23 RCTs and 5 meta-analyses examined
initial monotherapy of adults with generalized-onset
tonic clonic seizures
Division of trials
Class I (n=0)
Class II (n=0)
Class III (n=10):CBZ, GBP, LTG, OXC, PB, PHT,
TPM, VPA
Level A: None
Level B: None
Level C: CBZ*,LTG,OXC*,
PB, PHT*,TPM,VPA
Level D: GBP,VGB
Level E: Others
Level F: None
*=may aggravate tonic clonic seizures and more
commonly other generalized seizure types, should be
used with caution
Generalized Tonic Clonic Seizures: Adults
Recommendations
Generalized Tonic Clonic Seizures: Children
Available Evidence
A total of 20 RCTs examined initial monotherapy of children
with generalized onset tonic clonic seizures
Division of trials
Class I (n=0)
Class II (n=0)
Class III (n=14): CBZ, CLB, OXC, PB, PHT, TPM, VPA
Level A: None
Level B: None
Level C: CBZ*,PB, PHT*,TPM,VPA
Level D: OXC*
Level E: Others
Level F: None
*may aggravate tonic clonic seizures and more
commonly other generalized seizure types,
should be used with caution
Generalized Tonic Clonic Seizures: Children
Recommendations
Childhood Absence Epilepsy:
Available Evidence
A total of 6 RCTs examined initial monotherapy of
children with Childhood Absence Epilepsy
Division of trials
Class I (n=0)
Class II (n=0)
Class III (n=6) -3 Double Blinded
ETX, LTG, VPA
Level A: None
Level B: None
Level C: ESM, LTG, VPA
Level D: None
Level E: Others
Level F: CBZ, GBP, OXC, PB, PHT,TGB,VGB
Childhood Absence Epilepsy:
Recommendations
Initial Monotherapy
Idiopathic Localization Related
Epilepsy Syndromes:
Benign Epilepsy with
Centro-temporal Spikes (BECTS)
BECTS:
Available Evidence
A total of 3 RCTs examined initial monotherapy of children
with BECTS, 2 were DB
Division of trials
Class I (n=0)
Class II (n=0)
Class III (n=2)
Level A: None
Level B: None
Level C:CBZ, VPA
Level D: GBP,STM
Level E: Others
Level F: None
BECTS:
Recommendations
Initial Monotherapy
Idiopathic Generalized Epilepsy Syndromes:
Juvenile Myoclonic Epilepsy
Juvenile Myoclonic Epilepsy:
Available Evidence
A total of 0 RCTs examined initial monotherapy of
children with Juvenile Myoclonic Epilepsy
Division of trials
Class I (n=0)
Class II (n=0)
Class IIII (n=0)
Level A: None
Level B: None
Level C: None
Level D: CZP, LTG*, LEV, TPM, VPA, ZNS
Level E: Others
Level F: CBZ*, GBP, OXC*, PHT*, TGB, VGB
*may aggravate myoclonic seizure types, should be used with caution
Juvenile Myoclonic Epilepsy :
Recommendations
Juvenile myoclonic epilepsy
Drugs to be avoided
Clinical evidence has been provided that PHT, CBZ,
OXC, VGB, TGB, GBP (PRE?) may aggravate
absence and myoclonic seizures
LTG has been shown to aggravate severe
myoclonic epilepsies in infancy and in JME
Level of Evidence III-IV,
Recommendation C
Summary of Evidence and Recommendations
Partial onset seizures
Seizure
type or
epilepsy
syndrom
e
Class
I
Class
II
Class
III
Level of efficacy and effectiveness
evidence
(in alphabetical order)
POS:
Adults
2 1 30 Level A: CBZ, PHT, (LEV)
Level B: VPA
Level C: GBP, LTG, OXC, PB, TPM, VGB
POS:
Children
1 0 17 Level A: OXC
Level B: None
Level C: CBZ, PB, PHT, TPM, VPA
POS:
Elderly
1 1 2 Level A: GBP, LTG
Level B: None
Level C: CBZ
Summary of Evidence and Recommendations
Generalized onset seizures
Seizure
type or
epilepsy
syndrome
Class
I
Class
II
Class
III
Level of efficacy and effectiveness
evidence
(in alphabetical order)
GTC:
Adults
0 0 23 Level A: None
Level B: None
Level C: CBZ, LTG, OXC, PB, PHT, TPM,
VPA
GTC:
Children
0 0 14 Level A: None
Level B: None
Level C: CBZ, PB, PHT, TPM, VPA
Absence
seizures
0 0 6 Level A: None
Level B: None
Level C: ESM, LTG, VPA
Summary of Evidence and Recommendations
Epilepsy syndromes
Seizure
type or
epilepsy
syndrom
e
Class
I
Clas
s II
Clas
s III
Level of efficacy and effectiveness
evidence
(in alphabetical order)
BECTS 0 0 2 Level A: None
Level B: None
Level C: CBZ, VPA
JME 0 0 0 Level A: None
Level B: None
Level C: None
Variables that affect initial AED selection
AED-specific variables Patient-specific
variables
Nation-specific variables
Seizure type or
epilepsy syndrome
specific efficacy or
effectiveness
Dose-dependent
adverse effects
Idiosyncratic reactions
Chronic toxicities
Teratogenicity
Carcinogenicity
Pharmacokinetics
Interaction potential
Formulations
Genetic background
Age
Gender
Comedications
Comorbidities
Insurance coverage
Ability to swallow
pills/tablets
AED availability
AED cost
Insurance coverage
Participants in the ILAE Subcommission on
Antiepileptic Drug Guidelines
Elinor Ben-Menachem, Chairman
Tracy Glauser, USA
Blaise Bourgeois, USA
David Chadwick, UK
Avital Cnaan, USA
Carlos Guerreiro, Brazil
Reetta Kalviainen, Finland
Richard Mattson, USA
Emilio Perruca, Italy
Torbjrn Tomson, Sweden
Вам также может понравиться
- Evidence-Based Guidelines For The Treatment of Epileptic Seizures With AedsДокумент43 страницыEvidence-Based Guidelines For The Treatment of Epileptic Seizures With Aedsleonaldy sintesahealthОценок пока нет
- EBP Process: Ask Clinical Questions & Search EvidenceДокумент24 страницыEBP Process: Ask Clinical Questions & Search EvidenceMelda Amalia SikumbangОценок пока нет
- Introduction - Prof SudigdoДокумент49 страницIntroduction - Prof Sudigdoyulia fatma nstОценок пока нет
- Biostatistics ABSITE Review SeriesДокумент30 страницBiostatistics ABSITE Review SeriesSarah Chamas-AbdullaОценок пока нет
- Intervention Study Mayxay Laos 2009Документ72 страницыIntervention Study Mayxay Laos 2009Kausal VermaОценок пока нет
- EBM Prof Darwin 1. Introduction - Prof SudigdoДокумент49 страницEBM Prof Darwin 1. Introduction - Prof SudigdoMelda Amalia Sikumbang100% (1)
- What Study Design Should I Choose Caroline SabinДокумент70 страницWhat Study Design Should I Choose Caroline SabinaurielleОценок пока нет
- SAS Clinical Trails Phase-IДокумент70 страницSAS Clinical Trails Phase-IAnand KumarОценок пока нет
- $EBM Prof Darwin 1. Introduction - Prof SudigdoДокумент49 страниц$EBM Prof Darwin 1. Introduction - Prof SudigdoJunaida RahmiОценок пока нет
- Experimental Design RCTДокумент42 страницыExperimental Design RCTMeta M PurnamaОценок пока нет
- EBM TerapiДокумент23 страницыEBM TerapiArum Ardisa RiniОценок пока нет
- Triazole Antifungal Therapeutic Drug Monitoring: ECIL 6 MeetingДокумент76 страницTriazole Antifungal Therapeutic Drug Monitoring: ECIL 6 MeetingBengt HörbergОценок пока нет
- Understanding the Role of Laboratory Tests in Clinical PracticeДокумент43 страницыUnderstanding the Role of Laboratory Tests in Clinical Practicehendra2darmawanОценок пока нет
- Evidence-Based Medicine in Clinical PracticeДокумент42 страницыEvidence-Based Medicine in Clinical PracticeNur Rahmah KurniantiОценок пока нет
- DPS: Evidence Based Medicine and Clinical Epidemiology: Types of Research QuestionДокумент37 страницDPS: Evidence Based Medicine and Clinical Epidemiology: Types of Research Questionarjun.k5796Оценок пока нет
- Lecture 1 - IntroductionДокумент58 страницLecture 1 - IntroductionJames LittleОценок пока нет
- EBM CYCLE AND PICO QUESTIONДокумент40 страницEBM CYCLE AND PICO QUESTIONmuthia saniОценок пока нет
- Evidence Based Medicine: Kiki Lukman, Bagian Bedah Fakultas Kedokteran UNPAD/ RS DR Hasan Sadikin BandungДокумент70 страницEvidence Based Medicine: Kiki Lukman, Bagian Bedah Fakultas Kedokteran UNPAD/ RS DR Hasan Sadikin BandungOby BedahОценок пока нет
- GuidelinesДокумент27 страницGuidelinesUva TwittОценок пока нет
- Session 8Документ24 страницыSession 8Nyagawa GodkОценок пока нет
- Evidence-Based Practice: Picot AnalysisДокумент22 страницыEvidence-Based Practice: Picot Analysisdiana ratri100% (1)
- Critical Apprasial EbmДокумент64 страницыCritical Apprasial Ebmmisbah_mdОценок пока нет
- TB Triage Test Talk - McGill 2018 - NathavitharanaДокумент25 страницTB Triage Test Talk - McGill 2018 - NathavitharanaEllyana PerwitasariОценок пока нет
- Dr Yaser Adi's Evidence-Based Healthcare PresentationДокумент130 страницDr Yaser Adi's Evidence-Based Healthcare PresentationAnonymous hF5zAdvwCCОценок пока нет
- Evidence-Based Medicine,: Iwan Darmansjah Pusat Uji Klinik Obat, FkuiДокумент40 страницEvidence-Based Medicine,: Iwan Darmansjah Pusat Uji Klinik Obat, FkuiKastrat Lgm Fk UnismaОценок пока нет
- Randomized Controlled TrialДокумент38 страницRandomized Controlled TrialkundagolОценок пока нет
- Cukb Materi ViiДокумент34 страницыCukb Materi Viirandybayu235Оценок пока нет
- Evidence-Based Medicine: Lucman, Hakeymah Lunag, Tara Jane Magaoay, CheyserineДокумент71 страницаEvidence-Based Medicine: Lucman, Hakeymah Lunag, Tara Jane Magaoay, CheyserinereadmeamllionОценок пока нет
- Cardiovasular Health-KaveyДокумент59 страницCardiovasular Health-KaveyGhada HusseinОценок пока нет
- Multiple Myeloma Case Studies (Tandem 2017) (Final)Документ52 страницыMultiple Myeloma Case Studies (Tandem 2017) (Final)Syed Ali Akbar100% (1)
- CIDI-based Screening Scale For Bipolar Spectrum Disorders - : Clinical UtilityДокумент4 страницыCIDI-based Screening Scale For Bipolar Spectrum Disorders - : Clinical UtilityJagdishVankarОценок пока нет
- Ebm&Rm Dta Student 2023Документ14 страницEbm&Rm Dta Student 2023Sara Abdi OsmanОценок пока нет
- Celiac DiseaseДокумент33 страницыCeliac Diseasevalika_c100% (1)
- Critical Appraisal: Prof. Dr. Mohammad Hakimi, Spog (K), PHDДокумент37 страницCritical Appraisal: Prof. Dr. Mohammad Hakimi, Spog (K), PHDFebbty KuswantiОценок пока нет
- Critical Appraisal - Therapy - APTДокумент36 страницCritical Appraisal - Therapy - APTJason LiberiОценок пока нет
- Understanding Clinical TrialsДокумент41 страницаUnderstanding Clinical Trialsapi-37446750% (1)
- Evidence Based MedicineДокумент40 страницEvidence Based MedicinehafidzОценок пока нет
- An Evidenced-/based Approach To Treatment - Resistant Schizophrenia FlaumДокумент73 страницыAn Evidenced-/based Approach To Treatment - Resistant Schizophrenia FlaumElaОценок пока нет
- Evidence Based Medicine & Basic Critical AppraisalДокумент47 страницEvidence Based Medicine & Basic Critical AppraisalMohmmed Abu MahadyОценок пока нет
- Materi Kuliah - Introduction To EBMДокумент40 страницMateri Kuliah - Introduction To EBMRosi IndahОценок пока нет
- Introduction To Evidence-Based Case Report (EBCR)Документ48 страницIntroduction To Evidence-Based Case Report (EBCR)Mohammad Taufik PwОценок пока нет
- Oncology Nursing: An IntroductionДокумент46 страницOncology Nursing: An IntroductionLea Andreau Echavez SunoganОценок пока нет
- Critical Appraisal of Harm/Association StudiesДокумент26 страницCritical Appraisal of Harm/Association Studiesmirfanjee89Оценок пока нет
- EBM - Therapy: Gita Sekar PrihantiДокумент116 страницEBM - Therapy: Gita Sekar PrihantiMuhammida Fahriana100% (1)
- Critical Appraisal For RCT & Meta AnalisisДокумент8 страницCritical Appraisal For RCT & Meta AnalisisHanniОценок пока нет
- RCT Study DesignsДокумент47 страницRCT Study DesignsbramОценок пока нет
- Clinical - Trials - July 2019 PDFДокумент94 страницыClinical - Trials - July 2019 PDFAkansha GuptaОценок пока нет
- Diy RCT AppraisalДокумент43 страницыDiy RCT AppraisalimperiallightОценок пока нет
- Kul 7 - GATE Frame, Critical Appraisal DG Gambar FixДокумент81 страницаKul 7 - GATE Frame, Critical Appraisal DG Gambar FixbenzabensaaОценок пока нет
- CFPC SampsДокумент39 страницCFPC SampsSumer Chauhan100% (9)
- Evidence-Based Practice Resources For HINARI Users: (Module 7.2)Документ120 страницEvidence-Based Practice Resources For HINARI Users: (Module 7.2)thelordhaniОценок пока нет
- Evidence-Based Practice Resources For HINARI Users: (Module 7.2)Документ120 страницEvidence-Based Practice Resources For HINARI Users: (Module 7.2)thelordhaniОценок пока нет
- Good PICO QuestionДокумент122 страницыGood PICO QuestionFerdy Lainsamputty100% (1)
- PICO TX-punya BapaknyaДокумент56 страницPICO TX-punya BapaknyaIeie MawonОценок пока нет
- Complete Summary: Guideline TitleДокумент27 страницComplete Summary: Guideline TitleRudi ArdiyantoОценок пока нет
- Statistics Recalls and Some Other Quiez in StatisticsДокумент69 страницStatistics Recalls and Some Other Quiez in StatisticsLammia AlabsiОценок пока нет
- Fast Facts: Clinical Trials in Oncology: The fundamentals of design, conduct and interpretationОт EverandFast Facts: Clinical Trials in Oncology: The fundamentals of design, conduct and interpretationОценок пока нет
- FPG 007 CriticalCareinNeurology 2012Документ119 страницFPG 007 CriticalCareinNeurology 2012Pintu Mahligai100% (2)
- Who DMG PDFДокумент65 страницWho DMG PDFShelly Silvia BintangОценок пока нет
- Hubungan Pola Pemberian Asi Dengan Frekuensi Kejadian Diare Dan IspaДокумент9 страницHubungan Pola Pemberian Asi Dengan Frekuensi Kejadian Diare Dan IspaYuktika RiYuОценок пока нет
- F 3233 IDRT Acute Versus Chronic Invasive Fungal Rhinosinusitis A Case Control ST - PDF 4379Документ6 страницF 3233 IDRT Acute Versus Chronic Invasive Fungal Rhinosinusitis A Case Control ST - PDF 4379korokoro12Оценок пока нет
- Pi Is 1546144011005473Документ15 страницPi Is 1546144011005473korokoro12Оценок пока нет
- Ann Med Health Sci ResДокумент4 страницыAnn Med Health Sci Reskorokoro12Оценок пока нет
- 2011 ArticleДокумент5 страниц2011 ArticleZafira BustamОценок пока нет
- 2011 ArticleДокумент5 страниц2011 ArticleZafira BustamОценок пока нет
- Genotype-Phenotype Correlation, and Phenotypic HeterogeneityДокумент9 страницGenotype-Phenotype Correlation, and Phenotypic Heterogeneitykorokoro12Оценок пока нет
- Localized Aggressive Periodontitis in A Six-Year-Old: A Case ReportДокумент7 страницLocalized Aggressive Periodontitis in A Six-Year-Old: A Case Reportkorokoro12Оценок пока нет
- 588 PDFДокумент4 страницы588 PDFkorokoro12Оценок пока нет
- Diabetes GestacionalДокумент12 страницDiabetes GestacionalLuisa JaraОценок пока нет
- Who DMG PDFДокумент65 страницWho DMG PDFShelly Silvia BintangОценок пока нет
- Tes Diagnostik Cepat Utk MalariaДокумент5 страницTes Diagnostik Cepat Utk MalariaBeatrix KumowalОценок пока нет
- 588 PDFДокумент4 страницы588 PDFkorokoro12Оценок пока нет
- 2008-Antianxiety 2Документ43 страницы2008-Antianxiety 2korokoro12Оценок пока нет
- Nej MV CM 0904397Документ3 страницыNej MV CM 0904397korokoro12Оценок пока нет
- Pi Is 1546144011005473Документ15 страницPi Is 1546144011005473korokoro12Оценок пока нет
- P ('t':'3', 'I':'668634596') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)Документ11 страницP ('t':'3', 'I':'668634596') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)korokoro12Оценок пока нет
- Observational Study - Content Analysis - Genuinofinal FinalДокумент11 страницObservational Study - Content Analysis - Genuinofinal FinalIsaiah Win FloresОценок пока нет
- Percutaneous Needle Aspiration Versus Catheter Drainage in Treating Hepatic AbscessДокумент8 страницPercutaneous Needle Aspiration Versus Catheter Drainage in Treating Hepatic AbscessRatna TriasnawatiОценок пока нет
- Scope of Journal of Neonatal SurgeryДокумент2 страницыScope of Journal of Neonatal SurgeryMuhammad Bilal MirzaОценок пока нет
- Omura 2017Документ9 страницOmura 2017Wijah watiОценок пока нет
- The Effectiveness of Shoe Insoles For The Prevention and Treatment of Low BackДокумент19 страницThe Effectiveness of Shoe Insoles For The Prevention and Treatment of Low BackhasОценок пока нет
- Evidence Based Ophthalmology: Noel D. Atienza, MD, MSCДокумент59 страницEvidence Based Ophthalmology: Noel D. Atienza, MD, MSCJanBarlaanОценок пока нет
- Dupilumab significantly alleviates atopic dermatitis symptoms in meta-analysisДокумент15 страницDupilumab significantly alleviates atopic dermatitis symptoms in meta-analysisKoas PatoОценок пока нет
- Can Cognitive Therapy Be Conducted by Computers. Eells, Barrett, Wrigh, ThaseДокумент7 страницCan Cognitive Therapy Be Conducted by Computers. Eells, Barrett, Wrigh, ThaseAylin Lidsay Feria GОценок пока нет
- Promoting Community Reintegration Using Narratives and Skills Building For Young Adults With Stroke: A Protocol For A Randomised Controlled TrialДокумент8 страницPromoting Community Reintegration Using Narratives and Skills Building For Young Adults With Stroke: A Protocol For A Randomised Controlled TrialMAHESH KOUJALAGIОценок пока нет
- The Case Formulation Approach To Psychotherapy Research RevisitedДокумент22 страницыThe Case Formulation Approach To Psychotherapy Research Revisitedferreira.pipe3240Оценок пока нет
- Gina 2009Документ28 страницGina 2009Williams MenaОценок пока нет
- Immediate Versus Delayed Loading 2Документ8 страницImmediate Versus Delayed Loading 2fulmanti deviОценок пока нет
- The BIRD Recommendations On The Use of Ivermectin For Covid-19Документ99 страницThe BIRD Recommendations On The Use of Ivermectin For Covid-19Mario Manuel Fiorentino FerreyrosОценок пока нет
- Critical AppraisalДокумент17 страницCritical Appraisallowella100% (3)
- Weisz Et Al.2017 PDFДокумент39 страницWeisz Et Al.2017 PDFDaria PasailaОценок пока нет
- 2022 A Systematic Review and Meta-Analysis of 19 Randomized Controlled Trials of Iguratimod Combined With Other Therapies For Sjogren's SyndromeДокумент16 страниц2022 A Systematic Review and Meta-Analysis of 19 Randomized Controlled Trials of Iguratimod Combined With Other Therapies For Sjogren's SyndromemrsilОценок пока нет
- Discoloration of Teeth After Avulsion and Replantation - Results From A Multicenter Randomized Controlled Trial Peter F. Day PDFДокумент6 страницDiscoloration of Teeth After Avulsion and Replantation - Results From A Multicenter Randomized Controlled Trial Peter F. Day PDFKarina Delgado SantanaОценок пока нет
- All Ic AppendДокумент73 страницыAll Ic AppendSlaviša KovačevićОценок пока нет
- Negative Pressure Wound TherapyДокумент505 страницNegative Pressure Wound TherapyWario FrancoОценок пока нет
- Functional Exercise After Total Hip Replacement FE PDFДокумент8 страницFunctional Exercise After Total Hip Replacement FE PDFRizqy DwitamaОценок пока нет
- The Role of Complementary and Alternative Therapies in Managing Rheumatoid ArthritisДокумент1 страницаThe Role of Complementary and Alternative Therapies in Managing Rheumatoid Arthritisjelena_bojovic1Оценок пока нет
- Protocol SLR 53481 - Andreas TzeremesДокумент11 страницProtocol SLR 53481 - Andreas TzeremesAndreasОценок пока нет
- Actions To Increase Knowledge About Age-Related Fertility Decline in WomenДокумент9 страницActions To Increase Knowledge About Age-Related Fertility Decline in Womenrais123Оценок пока нет
- Econsort GuidelinesДокумент5 страницEconsort GuidelinesHansОценок пока нет
- Intraarterial Thrombolysis Techniques and Ongoing TrialsДокумент10 страницIntraarterial Thrombolysis Techniques and Ongoing TrialsIustitia Septuaginta SambenОценок пока нет
- Aquatic Therapy For MsДокумент7 страницAquatic Therapy For MsmadeОценок пока нет
- Sepsis in Peg Rcog PDFДокумент14 страницSepsis in Peg Rcog PDFSapna SОценок пока нет
- Sterile Processing in Low - and Middle-Income CountriesДокумент11 страницSterile Processing in Low - and Middle-Income Countriesmotoy asaph musanОценок пока нет
- Response: A Behavioural Insights Checklist For Designing Effective Communications Practitioners' PlaybookДокумент24 страницыResponse: A Behavioural Insights Checklist For Designing Effective Communications Practitioners' PlaybookAbhijeet NagpalОценок пока нет
- Aas 13501Документ13 страницAas 13501Felipe FernandesОценок пока нет