Академический Документы
Профессиональный Документы
Культура Документы
Vollhardt 6e Lecture PowerPoints - Chapter 12
Загружено:
superfr3shmАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Vollhardt 6e Lecture PowerPoints - Chapter 12
Загружено:
superfr3shmАвторское право:
Доступные форматы
CHAPTER 12
Reactions of Alkenes
Why Addition Reactions Proceed:
Thermodynamic Feasibility
12-1
Because the C-C bond is relatively weak, alkene chemistry is
dominated by its reactions.
The addition of a reagent, A-B, to give a saturated compound is
the most common transformation of an alkene.
H
o
for the above reaction can be estimated from the relevant
bond energies:
H
o
= (DH
o
bond
+ DH
o
A-B
) (DH
o
C-A
+ DH
o
C-B
)
Most additions to alkenes should proceed to products with the
release of energy.
Catalytic Hydrogenation 12-2
Hydrogenation takes place on the surface of a
heterogeneous catalyst.
In the absence of a catalyst, hydrogenations of alkenes, although
exothermic, do not spontaneously occur, even at high
temperatures.
In the presence of a catalyst, the same hydrogenations proceed
at a steady rate, even at room temperature.
The most frequently used catalysts for hydrogenation reactions
are:
Palladium dispersed on carbon (Pd-C)
Collodial platinum (Adams catalyst, PtO
2
)
Nickel (Raney nickel, Ra-Ni)
The primary function of a catalyst in hydrogenation reactions is to
provide metal-bound hydrogen atoms on the catalyst surface.
Common solvents used for hydrogenations include methanol,
ethanol, acetic acid, and ethyl acetate.
Hydrogenation is stereospecific.
During a hydrogenation reaction, both atoms of hydrogen are
added to the same face of the double bond (syn addition).
In the absence of steric hindrance, addition to either face of the
double bond can occur with equal probability which results in a
racemic mixture of products.
Nucleophilic Character of the Pi Bond: Electrophilic
Addition of Hydrogen Halides
12-3
The electrons of a double bond are more loosely held than those
of the bond.
As a result, the electrons, which extend above and below the
molecular plane of the alkene, can act as a nucleophile in a
manner similar to that of more typical Lewis bases.
2,3-dimethylbutene
The electrophilic addition reactions of alkenes can be both
regioselective and stereospecific.
Electrophilic attack by protons gives carbocations.
A strong acid may add a proton to a double bond to give a
carbocation.
This reaction is simply the reverse of the last step in an E1
elimination reaction and has the same transition state.
At low temperatures and with a good nucleophile, an electrophilic
addition product is formed.
Typically, the gaseous HX (HCl, HBr, or HI) is bubbled through the
pure or dissolved alkene. The reaction can also be carried out in a
solvent such as acetic acid.
The Markovnikov rule predicts regioselectivity in
electrophilic additions.
The only product formed during the reaction of propene with HCl
is 2-chloropropane:
Other addition reactions show similar results:
If the carbon atoms participating in the double bond are not
equally substituted, the proton from the hydrogen halide attaches
itself to the less substituted carbon.
As a result, the halogen attaches to the more substituted carbon.
This result is known as Markovnikovs rule and is based on the
stability of the carbocation formed by the addition of the proton.
Markovnikovs rule can also be stated:
HX adds to unsymmetric alkenes in a way that the initial
protonation gives the more stable carbocation.
Product mixtures will be formed from alkenes that are similarly
substituted at both sp
2
carbon atoms.
If addition to an achiral alkene generates a chiral product, a
racemic mixture will be obtained.
Carbocation rearrangements may follow
electrophilic addition.
In the absence of a good nucleophile, a rearrangement of the
carbocation may occur prior to the addition of the nucleophile.
An example of such a rearrangement is the addition of
trifluoroacetic acid to 3-methyl-1-butene, where a hydride shift
converts a secondary carbocation into a more stable tertiary
carbocation:
The extent of carbocation rearrangement depends upon:
alkene structure
solvent
strength and concentration of nucleophile
temperature
Rearrangements are generally favored under strongly acidic,
nucleophile-deficient conditions.
Alcohol Synthesis by Electrophilic Hydration:
Thermodynamic Control
12-4
When other nucleophiles are present, they may also attack the
intermediate carbocation.
Electrophilic hydration results when an alkene is exposed to an
aqueous solution of sulfuric acid (HSO
4
-
is a poor nucleophile).
The addition of water by electrophilic hydration follows
Markovnikovs rule, however carbocation rearrangements can
occur because water is a poor nucleophile.
The electrophilic hydration process is the reverse of the acid-
induced elimination of water (dehydration) of alcohols previously
discussed.
Alkene hydration and alcohol dehydration are
equilibrium processes.
In the absence of protons, alkenes are stable in water.
The position of the equilibrium in the hydration reaction can be
changed by adjusting the reaction conditions.
All steps are reversible in the hydration
of alkenes. The proton serves as a
catalyst only: it is regenerated in the
reaction.
The reversibility of alkene protonation leads to
alkene equilibration.
Protonation-deprotonation reactions may interconvert related
alkenes and produce an equilibrium mixture of isomers. Under
these conditions, a reaction is said to be under thermodynamic
control.
This mechanism can convert less stable alkenes into their more
stable isomers:
Electrophilic Addition of Halogens to Alkenes 12-5
Halogen molecules also act as electrophiles with alkenes giving
vicinal dihalides.
The reaction with bromine results in a color change from red to
colorless, which is sometimes used as a test for unsaturation.
Halogenations are best carried out at or below room temperature
and in inert halogenated solvents (i.e. halomethanes)
Electrophilic Addition of Halogens to Alkenes 12-5
Bromination takes place through anti addition.
Consider the bromination of cyclohexene. No cis-1,2-
dibromocyclohexane is formed.
Only anti addition is observed. The product is racemic since the
initial attack of bromine can occur with equal probability at either
face of the cyclohexene.
With acyclic alkenes the reaction is cleanly stereospecific:
Cyclic bromonium ions explain the stereochemistry.
The polarizability of the Br-Br bond allows heterolytic cleavage
when attacked by a nucleophile, forming a cyclic bromonium ion:
The bridging bromine atoms serves as the leaving group as the
bromonium ion is attacked from the bottom by a Br
-
ion.
In symmetric bromonium
ions, attack is equally
probable at either carbon
atom leading to racemic
or meso products.
The Generality of Electrophilic Addition 12-6
The bromonium ion can be trapped by other
nucleophiles.
Bromonation of cyclopentene using water as the solvent gives the
vicinal bromoalcohol (bromohydrin).
The water molecule is added anti to the bromine atom and the
other product is HBr.
Vicinal haloalcohols are useful synthetic intermediates.
Vicinal haloethers can be produced if an alcohol is used as the
solvent, rather than water.
Halonium ion opening can be regioselective.
Mixed additions to double bonds can be regioselective:
The nucleophile attacks the more highly substituted carbon of the
bromonium ion, because it is more positively polarized.
Electrophilic additions of unsymmetric reagents add in a
Markovnikov-like fashion: The electrophilic unit becomes attached
to the less substituted carbon of the double bond.
Mixtures of products are formed only when the two carbons are
not sufficiently differentiated.
Reagents of the type A-B, in which A acts as the electrophile, A
+
,
and B the nucleophile, B
-
, can undergo stereo- and regiospecific
addition reactions to alkenes:
Oxymercuration-Demercuration: A Special
Electrophilic Addition
12-7
The electrophilic addition of a mercuric salt to an alkene is called
mercuration. The product formed is known as an alkylmercury
derivative.
A reaction sequence known as oxymercuration-demercuration is
a useful alternative to acid-catalyzed hydration:
Oxymercuration is anti stereospecific and regioselective.
The alcohol obtained from oxymercuration-demercuration is the
same as that obtained from Markovnikov hydration, however,
since no carbocation is involved in the reaction mechanism,
rearrangements of the transition state do not occur.
Oxymercuration-demercuration in an alcohol solvent yields an
ether:
Hydroboration-Oxidation: A Stereospecific
Anti-Markovnikov Hydration
12-8
The boron-hydrogen bond adds across double
bonds.
Borane, BH
3
, adds to double bonds without catalytic activation:
The borane is
commercially available
in an ether-
tetrahydrofuran
solvent.
Because the borane is electron poor, and the alkene is electron
rich, an initial Lewis acid-base complex similar to the bromonium
ion can form:
Because of the four center transition state, the addition reaction is
syn. All three B-H bonds can react.
Hydroboration is regioselective as well as stereospecific (syn
addition).
Here, steric factors are more important than electronic factors.
The boron binds to the less hindered (substituted) carbon.
The oxidation of alkylboranes gives alcohols.
The oxidation of a trialkylborane by hydrogen peroxide produces
an alcohol in which the hydroxyl group has replaced the boron
atom.
In this reaction, the hydroxyl group ends up at the less
substituted carbon: an anti-Markovnikov addition.
During the oxidation, an alkyl group migrates with its electron
pair (with retention of configuration) to the neighboring oxygen
atom.
After all three alkyl groups have migrated to oxygen atoms, the
trialkyl borate is hydrolyzed by base to the alcohol and sodium
borate.
Hydroboration-oxidation of alkenes allows stereospecific and
regioselective synthesis of alcohols.
The reaction sequence exhibits anti-Markovnikov regioselectivity
which complements acid-catalyzed hydration and oxymercuration-
demercuration.
The reaction mechanism does not involve a carbocation and thus
rearrangements are not observed.
Diazomethane, Carbenes and Cyclopropane
Synthesis
12-9
Cyclopropanes can be readily prepared by the addition of a
carbene to the double bond of an alkene.
A carbene has the general structure, R
2
C:, in which the central
carbon is surrounded by six electrons (sextet), and is thus
electron deficient.
The electron-deficient carbene readily adds to an electron rich
alkene.
Diazomethane forms methylene, which converts
alkenes into cyclopropanes.
The highly reactive species methylene, H
2
C: (the simplest
carbene) can be produced from the decomposition of
diazomethane:
When methylene is generated in the presence of an alkene, an
addition reaction occurs producing a cyclopropane. This reaction
is usually stereospecific, with retention of the original double bond
configuration.
Halogenated carbenes and carbenoids also give
cyclepropanes.
Halogenated carbenes, prepared from halomethanes, can also be
used to synthesize cyclopropanes.
Treatment of trichloromethane (chloroform) with strong base
causes an elimination reaction in which both a proton and a
chlorine atom are removed from the same carbon.
The resulting product is a dichlorocarbene which reacts with
alkenes to produce cyclopropanes.
To avoid the hazards associated with diazomethane preparation,
an alternate route using diiodomethane and zinc (Simmons-Smith
reagent) to produce ICH
2
ZnI is used.
This substance is an example of a carbenoid, a carbenelike
substance that converts alkenes into cyclopropanes
stereospecifically.
Oxacyclopropane (Epoxide) Synthesis:
Epoxidation by Peroxycarboxylic Acids
12-10
Oxacyclopropanes contain a single oxygen atom connected to two
carbons to form a three-membered ring.
Oxacyclopropanes may be converted into vicinal anti diols.
Peroxycarboxylic acids deliver oxygen atoms to
double bonds.
Peroxycarboxylic acids have the general formula:
These compounds react with double bonds because one of the
oxygen atoms is electrophilic.
The resulting products are an oxacyclopropane and a carboxylic
acid.
This reaction is referred to as an epoxidation. The older common
name of an oxacyclopropane was an epoxide.
Commonly used peroxycaraboxylic acids for this reaction are
meta-chloroperoxybenzoic acid (MCPBA) which is somewhat
shock sensitive, and magnesium monoperoxyphthalate (MMPP).
The mechanism of this epoxidation reaction involves a cyclic
transition state:
The peroxycarboxylic acid reactivity with double bonds increases
with alkyl substitution, allowing for selective oxidations:
Hydrolysis of oxacyclopropanes furnishes the
products of anti dihydroxylation of an alkene.
Ring opening of oxacyclopropanes with water produces anti vicinal
diols.
Vicinal Syn Dihydroxylation with Osmium
Tetroxide
12-11
The reaction of osmium tetroxide with alkenes yields syn vicinal
diols in a two step process:
The reaction mechanism involves the concerted addition of the
osmium tetroxide to the bond of the alkene:
Catalytic amounts of osmium tetroxide in the presence of an
oxidizing agent (H
2
O
2
) to regenerate the spent osmium tetroxide
are often used, due to the expense and toxicity of OsO
4
.
An older reagent for vicinal syn dihydroxylation of alkenes is
KMnO
4
.
This reagent is less useful than OsO
4
because of its tendency
towards overoxidation.
The deep purple KMnO
4
is converted into a brown precipitate,
(MnO
2
) during the reaction, which can serve as a useful test for
the presence of alkenes.
Oxidative Cleavage: Ozonolysis 12-12
The mildest reagent capable of breaking both the and bonds
in a double bond is ozone, O
3
. This process is known as
ozonolysis.
Ozone is produced by an electrical discharge in dry oxygen in a
instrument called an ozonator.
The initial product of the reaction of ozone with an alkene is an
ozonide which is then directly reduced to two carbonyl products.
The mechanism of ozonolysis proceeds through a molozonide,
which breaks apart into two fragments, which then recombine to
form the ozonide:
Radical Additions: Anti-Markovnikov
Product Formation
12-13
Hydrogen bromide can add to alkenes in anti-
Markovnikov fashion: a change in mechanism.
The reaction products from the treatment of 1-butene with HBr
depend upon the presence or absence of molecular oxygen in the
reaction mixture:
In the presence of oxygen, a radical chain sequence mechanism
leads to the anti-Markovnikov product.
Small amounts of peroxides (RO-OR) are formed in alkene
samples stored in the presence of air (O
2
).
The peroxides initiate the radical chain sequence mechanism,
which is much faster than the ionic mechanism operating in the
absence of peroxides.
The halogens attack is regioselective, generating the more stable
secondary radical rather than the primary one.
The alkyl radical subsequently abstracts a hydrogen from HBr
which regenerates the chain-carrying bromine atom.
Both propagation steps are exothermic.
Termination is by radical recombination or by some other removal
of the chain carriers.
Commonly used peroxides for initiating radical additions include:
Are radical additions general?
HCl and HI do not give anti-Markovnikov addition products with
alkenes. The chain propagation steps involving these hydrogen
halides are endothermic, which leads to very slow reactions and
chain termination.
HCl and HI give Markovnikov products by ionic mechanisms
regardless of the presence of radicals.
Other reagents, such as thiols, do undergo successful radical
additions to alkenes:
Dimerization, Oligomerization, and
Polymerization of Alkenes
12-14
Alkenes can react with one another in the presence of an
appropriate catalyst: an acid, a radical, a base, or a transition
metal.
Polymer synthesis is of great industrial importance:
Carbocations attack pi bonds.
Protonation of 2-methylpropene by hot aqueous sulfuric acid
leads to the formation of two dimers:
The initial protonation produces a 1,1-dimethylethyl (tert-butyl)
cation which then attacks the double bond of a second 2-
methylpropene molecule.
The cation addition proceeds according to the Markovnikov rule to
generate the more stable carbocation.
Deprotonation of the addition product from either adjacent carbon
leads to a mixture of two products.
Repeated attack can lead to oligomerization and
polymerization.
When 2-methylpropene is treated with mineral acid under more
stringent conditions, higher oligiomers can be obtained through
repeated addition reactions:
At higher temperatures, polymers containing many subunits are
formed.
Synthesis of Polymers 12-15
Polymerization reactions can be categorized as cationic, radical,
anionic, and metal catalyzed.
Acid-catalyzed cationic polymerizations have already been
covered. Initiators include H
2
SO
4
, HF, and BF
3
.
Radical polymerizations lead to commercially useful
materials.
The polymerization of ethene in the presence of an organic
peroxide at high pressures and temperatures proceeds by a
radical polymerization process.
Polyethene (polyethylene) polymerized in this way is actually a
branched polymer. Branching occurs as a result of hydrogen
abstraction along the growing chain by another radical center.
The average molecular weight of polyethene is almost 1 million.
Polychloroethene (PVC or polyvinylchloride) is a polymer of
chloroethene (vinyl chloride).
The peroxide initiator and the intermediate radical chains add
only to the unsubstituted end of the monomer (producing the
most stable radical) which results in a very regular head-to-tail
structure of molecular weight over 1.5 million.
Pure PVC is fairly hard and brittle. It can be softened by the
addition of carboxylic acid esters (plasticizers) for use in elastic
materials such as vinyl leather, plastic covers, and garden hoses.
Polypropenenitrile (polyacrylonitrile) can be prepared from
propenenitrile (acrylonitrile) using hydrogen peroxide with FeSO
4
as a catalyst.
Polypropenenitrile, -(CH
2
CHCN)
n
-, also known as Orlon, is used to
make fibers.
Anionic polymerizations require initiation by bases.
Anionic polymerizations are initiated by strong bases such as
alkyllithiums, amides, alkoxides, and hydroxide.
The adhesive properties of Super Glue result from the hydroxide
initiated polymerization of 2-cyanopropenoate.
The electron withdrawing natures of the carbonyl and nitrile
groups create a partially positive carbon center at which the
hydroxide can initially attack.
The negative charge on the resulting anion is then resonance
stabilized by both the carbonyl and nitrile groups.
Metal-catalyzed polymerizations produce highly
regular chains.
Ziegler-Natta catalysts are important initiators for metal-
catalyzed polymerizations. They are typically made from titanium
tetrachloride and a trialkylaluminum such as Al(CH
2
CH
3
)
3
.
Polymers produced using a Ziegler-Natta catalyst are
characterized by regularity of construction and high linearity. This
results in much higher density and strength than similar polymers
obtained from radical polymerization.
Ethene: An Important Industrial Feedstock 12-16
Ethene is the basis for the production of polyethene
(polyethylene).
The major source of ethene is the pyrolysis of petroleum, or
hydrocarbons derived from natural gas.
Ethene is the starting material for the production of many other
industrial chemicals:
Alkenes in Nature: Insect Pheromones 12-17
Pheromones are chemical substances used for communication
within a living species. Pheromones are used for sex, trail, alarm,
and defense signaling, to name a few.
The sex attractant for the male silkworm moth is 10-trans-12-cis-
hexadecadien-1-ol (bombykol).
The natural pheromone is 10 billion
times more active in eliciting a response
than is the 10-cis-12-trans isomer, and
10 trillion times more active than the
trans, trans isomer.
Important Concepts 12
1. Double Bond Reactivity exothermic addition
reactions leading to saturated products
2. Hydrogenation of Alkenes immeasurably slow
unless a catalyst is used
Palladium on carbon, PtO
2
, Raney nickel
H
2
preferentially added to the least hindered face of
the double bond
3. Bond attacked by acid and electrophiles
If the initial intermediate is a carbocation, the more
highly substituted carbocation is formed
(Markovnikovs Rule).
If the initial intermediate is cyclic onium ion,
nucleophilic ring opening is at the more substituted
carbon (control of both regio- and stereochemistry).
Important Concepts 12
4. Hydroboration mechanistically between
hydrogenation and electrophilic addition
Step 1: complexation to boron
Step 2: concerted transfer of hydrogen to carbon
Hydroboration-oxidation: anti-Markovnikov hydration
of alkenes
5. Carbenes and Carbenoids useful for synthesis
of cyclopropanes from alkenes
6. Peroxycarboxylic Acids contains oxygen atom
transferable to alkenes to give oxacyclopropanes
(epoxidation)
7. Osmium Tetroxide addition to alkenes in a
concerted syn manner to give vicinal diols
Important Concepts 12
8. Ozonolysis when followed by reduction, yields
carbonyl compounds by cleavage of the double bond
9. Radical Chain Additions To Alkenes
Chain carrier adds to the bond to form the more
highly substituted radical
Allows for anti-Markovnikov hydrobromination of
alkenes
Allows for the addition of thiols and some
halomethanes
10. Polymers Alkenes react with themselves to form
polymers.
Initiation by charged species, radicals or some
transition metals
Вам также может понравиться
- Electrophilic Addition of Hydrogen HalidesДокумент16 страницElectrophilic Addition of Hydrogen HalidesDk Hazra HadzryaОценок пока нет
- 4.2 Hydration of AlkenesДокумент33 страницы4.2 Hydration of AlkenesDawit BirhanuОценок пока нет
- Chapter 8 Alkenes and Alkynes II4Документ7 страницChapter 8 Alkenes and Alkynes II4wimal nayanaОценок пока нет
- Regioselective Reactions: Regioselective Means That The Reaction Selectively Produces One Regioisomer As TheДокумент8 страницRegioselective Reactions: Regioselective Means That The Reaction Selectively Produces One Regioisomer As Therihana yadavОценок пока нет
- Electrophilic Addition of Alkenes NotesДокумент17 страницElectrophilic Addition of Alkenes NotesAnanda Vijayasarathy100% (1)
- Addition Reactions of Alkenes: Hydrogenation, Hydration, Halogenation, OzonolysisДокумент89 страницAddition Reactions of Alkenes: Hydrogenation, Hydration, Halogenation, OzonolysisasyaanazОценок пока нет
- Addition Reactions of AlkenesДокумент34 страницыAddition Reactions of AlkenesAlvis MwangiОценок пока нет
- Free Radical Substitution and Electrophilic AdditionДокумент17 страницFree Radical Substitution and Electrophilic Additionchicko33Оценок пока нет
- Organic Chemistry Alkynes ReactionsДокумент9 страницOrganic Chemistry Alkynes ReactionsAnthony KwofieОценок пока нет
- Reactions of AlkenesДокумент22 страницыReactions of Alkenesdela2Оценок пока нет
- AlkenesДокумент120 страницAlkenesVidhan PatniОценок пока нет
- AlkenesДокумент16 страницAlkenesAbhijeetОценок пока нет
- Rearrangement of Carbocations: Major MinorДокумент20 страницRearrangement of Carbocations: Major Minortarun kumarОценок пока нет
- Alkenes: Reactions and MechanismsДокумент45 страницAlkenes: Reactions and MechanismsBritney PattersonОценок пока нет
- Alkene and Alkyne Addition ReactionsДокумент28 страницAlkene and Alkyne Addition ReactionsNH Khánh NhiiОценок пока нет
- Reactions of Alkenes: Addition and HydrationДокумент34 страницыReactions of Alkenes: Addition and HydrationIamKasiraporn MahanilОценок пока нет
- Organic ReactionsДокумент44 страницыOrganic ReactionsJanhavi PatilОценок пока нет
- of HydrocarbonsДокумент45 страницof HydrocarbonsSneha KediaОценок пока нет
- Markov Niko VsДокумент9 страницMarkov Niko VsPipit Aditia ListiyaniОценок пока нет
- Production of HalogenoalkanesДокумент17 страницProduction of HalogenoalkanesMuhammad KalimОценок пока нет
- Organic Reactions and MechanismДокумент51 страницаOrganic Reactions and MechanismAbhay Kumar Nayak75% (8)
- Adisi Elektrofilik Alkena AlkunaДокумент37 страницAdisi Elektrofilik Alkena Alkunatutik kharismayantiОценок пока нет
- Organic MaterialДокумент15 страницOrganic MaterialAditya GathwalaОценок пока нет
- Synthesis of AlcoholsДокумент10 страницSynthesis of AlcoholsMustaffa MansoorОценок пока нет
- Organic Chemistry Chapter 8Документ41 страницаOrganic Chemistry Chapter 8채종희Оценок пока нет
- Chapter 4Документ43 страницыChapter 4민규강Оценок пока нет
- Alkene 6Документ5 страницAlkene 6Prazwal RegmiОценок пока нет
- Organic Chemistry II Chapter 22 Notes on Alpha Substitution ReactionsДокумент9 страницOrganic Chemistry II Chapter 22 Notes on Alpha Substitution ReactionsKuandi TanОценок пока нет
- AdisiДокумент107 страницAdisiazizgagabОценок пока нет
- Organic Chemistry Alkenes Reactions and SynthesisДокумент55 страницOrganic Chemistry Alkenes Reactions and Synthesis0JTINGОценок пока нет
- Part 1 Alkanes: Organic ChemistryДокумент5 страницPart 1 Alkanes: Organic ChemistryRuonan QinОценок пока нет
- Organic Chemistry Study SheetДокумент22 страницыOrganic Chemistry Study SheetJosephine Chen100% (1)
- Alkenes and AlkynesДокумент22 страницыAlkenes and AlkynesAyodele AdeyonuОценок пока нет
- HYDROBORATIONДокумент15 страницHYDROBORATIONArya NairОценок пока нет
- Alkenes and Alkynes: Structure and Physical PropertiesДокумент16 страницAlkenes and Alkynes: Structure and Physical PropertiesSaloni JainОценок пока нет
- Electrophilic Addition Reactions ExplainedДокумент30 страницElectrophilic Addition Reactions ExplainedBhumika ghimireОценок пока нет
- CIE AS Chemistry Organic ChemistryДокумент41 страницаCIE AS Chemistry Organic Chemistrykudec2008Оценок пока нет
- Electrophilic Addition of Hydrogen Bromide (HBR (Conc. Aq) and HBR (Gnon-Polar Solvent) ) To Form HalogenoalkanesДокумент4 страницыElectrophilic Addition of Hydrogen Bromide (HBR (Conc. Aq) and HBR (Gnon-Polar Solvent) ) To Form HalogenoalkaneskushanОценок пока нет
- Chapter 6. Addition ReactionДокумент15 страницChapter 6. Addition Reactionahmedmustefa773Оценок пока нет
- AlkenesДокумент27 страницAlkenesDo Thu HienОценок пока нет
- AlkenesДокумент11 страницAlkenesPhillimonОценок пока нет
- Lecture 5-AlkenesДокумент34 страницыLecture 5-Alkenesanasattiq078Оценок пока нет
- Addition ReactionДокумент34 страницыAddition Reactionmichot feleguОценок пока нет
- Chapter 10 Haloalkanes and HaloarenesДокумент19 страницChapter 10 Haloalkanes and HaloarenesSujithОценок пока нет
- Summary of Organic ReactionsДокумент21 страницаSummary of Organic ReactionsMarie St. LouisОценок пока нет
- Electrophilic Addition Reactions PDFДокумент6 страницElectrophilic Addition Reactions PDFMSMОценок пока нет
- Elimination and Addition ReactionДокумент8 страницElimination and Addition Reactionpbienizeg.23Оценок пока нет
- Poc Unit-5Документ9 страницPoc Unit-5Bintoo SharmaОценок пока нет
- HydroborationДокумент5 страницHydroborationTwasalifya SiwaleОценок пока нет
- Heloalkanes and HeloarenesДокумент8 страницHeloalkanes and HeloarenesPuneet K UppalОценок пока нет
- Summary of HaloalkaneДокумент10 страницSummary of HaloalkaneTai PanОценок пока нет
- Document 4Документ10 страницDocument 4nida shahbazОценок пока нет
- 2.11 MechanismДокумент38 страниц2.11 MechanismAmber Michaels100% (1)
- CAIE Chemistry A-Level: 15: Halogen CompoundsДокумент7 страницCAIE Chemistry A-Level: 15: Halogen CompoundsahumanbeinginearthОценок пока нет
- Organic Chemistry: Alkene NotesДокумент11 страницOrganic Chemistry: Alkene NotesDommie FranklinОценок пока нет
- Organic Chemistry 2013Документ41 страницаOrganic Chemistry 2013Claudia JaukinОценок пока нет
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersОт EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersОценок пока нет
- Biochem Sample Test-2-2016-AnswersДокумент6 страницBiochem Sample Test-2-2016-Answerssuperfr3shmОценок пока нет
- History 9-17Документ1 страницаHistory 9-17superfr3shmОценок пока нет
- History 131 2015 Exam I Essay PossibilitiesДокумент1 страницаHistory 131 2015 Exam I Essay Possibilitiessuperfr3shmОценок пока нет
- Vollhardt 6e Lecture PowerPoints - Chapter 13Документ47 страницVollhardt 6e Lecture PowerPoints - Chapter 13superfr3shmОценок пока нет
- Vollhardt 6e Lecture PowerPoints - Chapter 10Документ73 страницыVollhardt 6e Lecture PowerPoints - Chapter 10superfr3shm100% (1)
- Self Test Exam 1Документ1 страницаSelf Test Exam 1superfr3shmОценок пока нет
- Microbiology NotesДокумент4 страницыMicrobiology Notessuperfr3shmОценок пока нет
- HW CH16Документ12 страницHW CH16superfr3shmОценок пока нет
- Brittingham 1983Документ6 страницBrittingham 1983superfr3shmОценок пока нет
- Vollhardt 6e Lecture PowerPoints - Chapter 10Документ73 страницыVollhardt 6e Lecture PowerPoints - Chapter 10superfr3shm100% (1)
- Vollhardt 6e Lecture PowerPoints - Chapter 10Документ73 страницыVollhardt 6e Lecture PowerPoints - Chapter 10superfr3shm100% (1)
- Vollhardt 6e Lecture PowerPoints - Chapter 11Документ58 страницVollhardt 6e Lecture PowerPoints - Chapter 11superfr3shmОценок пока нет
- VDJ Recombination With Antibody StructureДокумент38 страницVDJ Recombination With Antibody Structuresuperfr3shmОценок пока нет
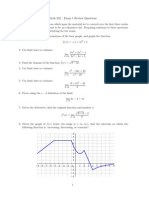
- Math 242: Exam 1 Review QuestionsДокумент3 страницыMath 242: Exam 1 Review Questionssuperfr3shmОценок пока нет
- Test 3 Study GuideДокумент1 страницаTest 3 Study Guidesuperfr3shmОценок пока нет
- Exp - 4 A, DistillationДокумент21 страницаExp - 4 A, Distillationsuperfr3shmОценок пока нет
- Reduction of N Cinnamylidene M NitroanilineДокумент9 страницReduction of N Cinnamylidene M Nitroanilinesuperfr3shmОценок пока нет
- Final Exam Formulas: Significance Test About A Population SlopeДокумент1 страницаFinal Exam Formulas: Significance Test About A Population Slopesuperfr3shmОценок пока нет
- The Genetics Revolution in The Life SciencesДокумент16 страницThe Genetics Revolution in The Life Sciencessuperfr3shmОценок пока нет
- KU Core CurriculumДокумент3 страницыKU Core Curriculumsuperfr3shmОценок пока нет
- Physics CH 13Документ20 страницPhysics CH 13superfr3shmОценок пока нет
- EXP 6 - SN2 ReactionДокумент17 страницEXP 6 - SN2 Reactionsuperfr3shmОценок пока нет
- Pie Acceptor Ligands AssignmentДокумент13 страницPie Acceptor Ligands AssignmentTaimoor Hassan KhanОценок пока нет
- Health Benefits of AntioxidantsДокумент3 страницыHealth Benefits of AntioxidantsYamile Gonzalez RodriguezОценок пока нет
- AntioksidanДокумент109 страницAntioksidanHasbi O'conner Part IIОценок пока нет
- HaloalkanesДокумент12 страницHaloalkanesMo_Bash1Оценок пока нет
- Abstract BookДокумент28 страницAbstract BookParjanya ShuklaОценок пока нет
- DR Duke Essential Herbs James A Duke, PHD (Orthomolecular Medicine)Документ203 страницыDR Duke Essential Herbs James A Duke, PHD (Orthomolecular Medicine)Ebook PDF86% (7)
- Hidrogen Plasma Reactors 1-s2.0-S036031992204839X-mainДокумент16 страницHidrogen Plasma Reactors 1-s2.0-S036031992204839X-mainsolutronicОценок пока нет
- Free RadicalsДокумент18 страницFree Radicalsswetha ramОценок пока нет
- Diet of ManДокумент20 страницDiet of Manstan_avesОценок пока нет
- Laser GraftingДокумент5 страницLaser GraftingpcnferreiraОценок пока нет
- Organic Chemistry MasterДокумент128 страницOrganic Chemistry MasterLeigh DensingОценок пока нет
- Benefits of Alkaline Water EbookДокумент58 страницBenefits of Alkaline Water Ebookanitabillion100% (3)
- Flame Retardancy of Silicone-Based MaterialsДокумент31 страницаFlame Retardancy of Silicone-Based Materialsdungnv2733100% (1)
- Oxid Antioxid Med Sci 201431Документ91 страницаOxid Antioxid Med Sci 201431Med AjОценок пока нет
- Antioxidant Properties of Different Fractions of Vitex Negundo Linn. - ScienceDirect PDFДокумент2 страницыAntioxidant Properties of Different Fractions of Vitex Negundo Linn. - ScienceDirect PDFCeline Jane DiazОценок пока нет
- Antioxidant and Antiinflammatory Properties of Curcumin PDFДокумент21 страницаAntioxidant and Antiinflammatory Properties of Curcumin PDFAisyahОценок пока нет
- Organic Reaction Mechanism PDFДокумент91 страницаOrganic Reaction Mechanism PDFShubhendu KarmakarОценок пока нет
- Chapter 6-10 PDFДокумент151 страницаChapter 6-10 PDFdushimirimana eliasОценок пока нет
- Super Immunity by Joel Fuhrman, M.D.Документ31 страницаSuper Immunity by Joel Fuhrman, M.D.HarperOne (an imprint of HarperCollins)89% (19)
- Oxidative Stress and AntioxidantsДокумент34 страницыOxidative Stress and AntioxidantsTauseefОценок пока нет
- Free RadicalsДокумент46 страницFree Radicalssakumar5678Оценок пока нет
- Kinetics of Radical PolymerizationДокумент75 страницKinetics of Radical Polymerizationania20011Оценок пока нет
- 6BBL0325 2 TheoriesofAgeingRandomDamageДокумент10 страниц6BBL0325 2 TheoriesofAgeingRandomDamageVaidehi KatariaОценок пока нет
- Engineering ChemistryДокумент60 страницEngineering ChemistryAditya ShindeОценок пока нет
- DPPH Radical Scavenging AssayДокумент20 страницDPPH Radical Scavenging AssayBeatriz FriasОценок пока нет
- 1998-Mechanistic Action of Phenolic Antioxidants in Polymers A ReviewДокумент22 страницы1998-Mechanistic Action of Phenolic Antioxidants in Polymers A ReviewSopan TambekarОценок пока нет
- (Oxidative Stress in Applied Basic Research and Clinical Practice) Josef Miller, Colleen G. Le Prell, Leonard Rybak (Eds.) - Free Radicals in ENT Pathology (2015, Humana Press) PDFДокумент501 страница(Oxidative Stress in Applied Basic Research and Clinical Practice) Josef Miller, Colleen G. Le Prell, Leonard Rybak (Eds.) - Free Radicals in ENT Pathology (2015, Humana Press) PDFBlebea CristinaОценок пока нет
- Photostability of Epinephrine Reduced by Bisulfite and Degradation ProductsДокумент7 страницPhotostability of Epinephrine Reduced by Bisulfite and Degradation ProductsAriel GarciaОценок пока нет
- Adsorption of Methyl Orange Using Self-Assembled Porous MicrosphereДокумент407 страницAdsorption of Methyl Orange Using Self-Assembled Porous MicrosphereHaroon RashidОценок пока нет
- Advantages of Techniques To Fortify Food Priducts With The Benefits of Fish OilДокумент17 страницAdvantages of Techniques To Fortify Food Priducts With The Benefits of Fish Oilaisyah_asyrafОценок пока нет