Академический Документы
Профессиональный Документы
Культура Документы
Wastewater Treatment: Physical and Chemical Process: Basic Concepts
Загружено:
VIRGILIO MANANGAN0 оценок0% нашли этот документ полезным (0 голосов)
78 просмотров49 страницMore than one treatment is needed to achieve the desired change in quality. In some cases treatment processes do not destroy the impurity but simply concentrate them in the form of sludge or effluent stream. Wastewater treatment is divided into three main processes. Physical processes comprising screening or straining, sedimentation, flocculation and filtration. Chemical treatment using adsorption, coagulation, ion exchange, precipitation. Biological treatment processes with dispersed growth system (activated s
Исходное описание:
Оригинальное название
Wastewater Treatment
Авторское право
© © All Rights Reserved
Доступные форматы
PPT, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документMore than one treatment is needed to achieve the desired change in quality. In some cases treatment processes do not destroy the impurity but simply concentrate them in the form of sludge or effluent stream. Wastewater treatment is divided into three main processes. Physical processes comprising screening or straining, sedimentation, flocculation and filtration. Chemical treatment using adsorption, coagulation, ion exchange, precipitation. Biological treatment processes with dispersed growth system (activated s
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PPT, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
78 просмотров49 страницWastewater Treatment: Physical and Chemical Process: Basic Concepts
Загружено:
VIRGILIO MANANGANMore than one treatment is needed to achieve the desired change in quality. In some cases treatment processes do not destroy the impurity but simply concentrate them in the form of sludge or effluent stream. Wastewater treatment is divided into three main processes. Physical processes comprising screening or straining, sedimentation, flocculation and filtration. Chemical treatment using adsorption, coagulation, ion exchange, precipitation. Biological treatment processes with dispersed growth system (activated s
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PPT, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 49
WASTEWATER TREATMENT:
PHYSICAL AND CHEMICAL
PROCESS
BASIC CONCEPTS:
In order to dispose off the waste water, it should
meet some standards set by local authorities. With
these standards wastewater should be treated.
More than one treatment is needed to achieve the
desired change in quality. Thus, it is a chain
processes operating in sequence.
In some cases treatment processes do not
destroy the impurity but simply concentrate
them in the form of sludge or effluent
stream.
Selection of proper method based on the
characteristics of wastewater is the key to
solving treatment problems.
Nature of Impurities
Rainwater, surface water, groundwater, seawater
can be considered as the different forms of some
basic material, varying in form and amount of
impurities. These impurities decide the level of
treatment provide for its use and disposal.
Municipal water has many types of impurities like floating
and large suspended solids(paper, rags, plastics, grit),
dissolved solids( organic and inorganic);Dissolved gases(
hydrogen sulfide, methane etc.) and
microorganisms(pathogen,bacteria and viruses)
Wastewater on the other hand can have much higher levels
of impurities as discussed because of the human organic
waste added to water along with detergents, pesticides, and
microorganism.
But wastewater can be called as water of different form if
the concentration of impurities is reduced, it can have
applications similar to water.
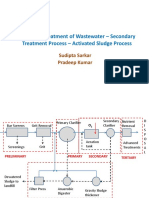
Different treatment processes
Wastewater treatment is divided into three main
processes.
1. Physical processes comprising screening or
straining, sedimentation, flocculation and
filtration.
2. Chemical treatment using adsorption,
coagulation, ion exchange, precipitation.
3. Biological treatment processes with dispersed
growth system( activated sludge, stabilization
ponds); fixed film reactors( biological filters
such as tricking filter)
Wastewater treatment processes normally
uses physical processes initially and later
chemical processes like precipitation. But if
it not sufficiently treated by the two
processes, the three can be used.
Raw sewage
P P
Sea Outfall
screening
Sedimentation
Raw sewage
P P B
P
Surface
water
Aerobic
oxidation
sedimentation
sedimentation
Screening
Raw
sewage
P P B C
P P
C
Sensitive
surface water
sedimentation
screening
Aerobic
oxidation
Phosphorus
removal
sedimentation
filtration
Disinfection
Note: P- Physical Process
C- Chemical Process
B- Biological Process
Wastewater treatment
process chain
Most of processes in wastewater treatment plant
as it is most cost effective compared to batch
process. However, batch process are preferred in
some cases where the quantity of waste is small
and the waste generation is not continuous.
Physical treatment processes
The main aim of physical treatment process
in a water treatment system is to protect the
main treatment systems from possible
damage or clogging. Various processes are
used to remove large floating and
suspended material.
Screening
The first operation in the physical treatment process. Micro
strainer with specially woven stainless steel meshes size
between 20 to 40 micrometer are usually used in water
treatment plants.
The nature and quantity of screening collected depend
upon the social, dietary, as well sewerage system of the
city. It also depends on the screening bar spacing, which
affect the loss of velocity head. This headloss indicates the
performance of a screen. On an average its quantity ranges
from .01 to .03 cu.m./1000 population per day.
Screening is unpleasant in nature with a strong
odor, particularly in high temperatures. Their
density is usually 1800-1900 kg/cu.m. with a solid
content of 15-30%
Sample problem:
A bar screen is installed in a wastewater treatment
plant receiving a daily peak flow of crude sewage
of 50,000 cu.m. Estimate headloss through the
screen and also the gross area of the screen.
Solution
Maximum flow, Q= 50,000 cu.m.=.5788 cu.m/s
Desired velocity through the screen, V,at ultimate flow= 0 .8m/s
Net area of screen opening required, A= .5788/.8 = 0.72 m/s
Since Q= V/A
Using rectangular bars in the screen having 1 cm width, 1 m height and
placed 5 cm clear spacing,
Number of bars required = .7235 * 100/5 = 14.46
Say 15 bars of 1 cm each
Total area required for screen = 0.7235 + (15 * 10^-2) = 0.87 square meter
Assuming that the inclination of the screen to the horizontal is at 60
degrees
The gross area of screen would be
= 0.87 * (square root of 3/ 2) = 1 square meter
Velocity through the clear screen, v = 0.8m/s
Velocity above the screen , u = 0.8 * 5/6 m/s = 0.67 m/s
Using the equation of continuity
Headloss through the screen is usually given as
= 0.0729 (v^2- u^2)
= 0.0729 (0.8^2 0.67^2)
= 0.014 m
If the screen openings are half plugged with screenings, leaves and
debris, the velocity through the screen is doubled.
v = 0.8 * 2 m/s
= 1.6 m/s
Maximum headloss = .0729 ( 1.6^2 0.67 ^2)
= 0.15m
At this maximum headloss the screen is to be cleaned.
In situ maceration of floating and large suspended solids can be
achieved by a comminutor or macerator in which the material is
trapped between teeth mounted on a slotted rotating drum and a
fixed comb.
Comminution and maceration
Grit removal
In most sewerage plant, considerable amount of sand and grit are
transported to the wastewater treatment plant. These materials arise
from roads and other paved areas. These two should be removed
earlier in order to prevent damage on the pumps and other mechanical
equipments.
Sedimentation
Is the separation of solids based on difference between densities of
solids and wastewater due to gravity.
Stokes law
Vs = (gd^2/18V) (Ss 1)
Where Vs = discrete particle terminal velocity; g is acceleration due to
gravity; d= diameter of the particle ; V = kinematic viscosity of water
Ss = specific gravity of the particle
Practical consideration in settlement of the discrete suspension
involves concept of ideal settling basin in which it is assumed that the
following conditions exists.
Quiescent settlement in the settling zone
Uniform flow through the settling zone
Uniform solids concentrations entering the settling zone.
Solids entering the sludge zone are not resuspended.
Horizontal and radial
flow units
Surface overflow rate 1- 1.5 m/h
Retention time 2 hr
Outlet weir loading < 12.5 cu.m / mh
Width : length
Rectangular units 1:4 to 1: 8
Vertical Flow units
Surface overflow rate 1- 1.8 m/h
Retention time 2-3 hr
Outlet weir loading < 12.5 cu.m /mh
Final settlement after
biological treatment
surface overflow rate 1.5 m/h
Retention time 2 hr
Outlet weir loading < 10 cu.m / mh
Typical design criteria for
sedimentation tanks.
Types of sedimentation tank
parameter Rectangular circular
Max. length 90m -
Max. width 30m -
depth 2- 2..5 2- 3.5 m
Range of
length/width ratio
1.5- 7.5
Range of length/
depth ratio
5 25
Bottom slope 1% 7.5 10% ( from periphery to center)
Max. diameter 30m
inlet multiple pipes on the central inlet pipe with concentric
width side with inlet baffle of diameter 15% of
baffle boards of depth 0.5 m and tank diameter and extending
0.8 m in front of the pipe 1m below surface
inlets and surface
for scum passover
Outlet Overflow weir with V-notches to peripheral weir provided with
Provide uniform flow at low heads. V- notch. Scum baffle
Scum baffles provide ahead of extending 0.3 m below
weir for wastewater installations water surface provided ahead
of effluent weir for
wastewater installation
Peak velocity Depends upon feed
Scraper arms velocity 0.2 m.min
Design features of wastewater sedimentation tank
Types of sedimentation tank
Typical sedimentation tanks: (a) rectangular horizontal flow tank; (b) circular, radial-flow tank; (c) hopper-bottomed, upward flow
tank
(a) Inlet zone at the central well, which has a round baffle plate, the
flow is established in a uniform radial direction so that short-circuiting
does not take place.
(b) Settling zone where settling is assumed to occur as the water
flows towards the outlet.
(c) Outlet zone in which the flow converges up and over the
decanting weirs.
(d) Sludge zone where settled material collects and is pumped out.
Important parts of a sedimentation tanks
Flocculation
This process is used when the small suspended solids having low specific
gravity and low settling velocity cannot be separated by sedimentation
easily. In wastewater treatment,this usually occurs particularly with
particles of less than 50 micrometer in size.
Advanced physical processes
These are used in order to get better
treatment efficiency. Some of the better
known operations used in advanced
treatment are ammonia stripping,
distillation, foam, fractionation,freezing.
Ammonia stripping
Is also known as air stripping of ammonia.
It is a modification of the aeration process
used for the removal of gases dissolved in
water.
Illustration of ammonia stripping
In the first phase, sodium hydroxide (NaOH) is added to the wastewater stream
just before being pumped into the stripping column. This raises the water pH
to the point where ammonium ions (NH 4 + ) are converted to ammonia gas.
Ammonia removal is enhanced by pumping the wastewater stream into the top
of the column which is packed with high surface area packing material. Air is
pumped into the bottom of the column. The ammonia carried by the air stream
is then transferred to liquid in the scrub column.
At the core of AmmEL-HC is a patented electrochemical reactor that converts
the ammonia dissolved in the scrubbing liquid to gaseous Nitrogen without
producing nitrates or greenhouse gases . Chemically inert and occupying an 80
per cent proportion of our atmosphere, gaseous nitrogen is the ideal end
product for safe dispersal into the environment.
Testing of the AmmEL-HC system has demonstrated its capabilities. As
shown below, a percentage removal ranging from 93% to 99% was achieved
throughout the test.
Other advantages of AmmEL-HC include:
Improves performance of existing biological treatment plants by
preventing ammonia toxicity in the activated sludge process
Less expensive than conventional processes, including biological
treatment
Does not convert ammonia to nitrate and does not produce greenhouse
gases
Small footprint - can reduce overall cost of land for new biological
treatment plants
Can operate intermittently without start-up delays
Fully automated - low maintenance
Remotely monitored
distillation
Is a unit operation in which the components of a liquid solution are
separated vaporization and condensation.
Foam fractionation
Is the separation of colloidal and suspended
material by floatation and dissolved organic
matter by adsorption.
freezing
Wastewater is sprayed into a chamber
operating under a vacuum. A portion of
wastewater gets evaporated and cooling
effect produces contaminant free ice
crystals in the remaining liquid.
Gas phase separation
Is a promising method for removal of
ammonia as gas. This method is based on
the development of selective permeable gas-
phase membranes.
Reverse osmosis
Is the process in which water is separated
from dissolved salts in the solution by
filtering through semi permeable membrane
at a pressure than the osmotic pressure
caused by the dissolved salts in wastewater.
Sorption
Conventional alum treatment for removal of
phosphate increases the concentration of
sulphate ions in the solution. To avoid this,
sorption is developed. Sorption is a process
of removing various forms of phosphate
without increasing the concentration of
sulphates.
Chemical treatment processes
Chemical precipitation- is the method of
addition of chemicals to the wastewater,
converting undesired soluble substances
into an insoluble precipitate which can be
removed easily and rapidly.
The undesired constituents generally found in wastewater are:
Suspended colloidal and supra colloidal solids and their associated
organic matter. They should be removed in order to avoid damage to
performance and reduction in efficiency.
Phosphorus
Toxic heavy metals and organics discharged from industrial plants and
premises into the municipal wastewater treatment plant.
coagulation
Is an important operation in removing colloidal solids. In
wastewater treatment, chemical such as lime, ferric salts
and commercial alum are used as coagulants. This process
removes suspended solids ( 60-80% ), BOD ( 50-70% )
phosphorus ( over 90%) and heavy metal ( over 80%)
Chemical clarification of wastewater can be combined with
the activated carbon adsorption process to provide
complete physical chemical wastewater treatment. the
use of chemical clarification has restricted application in
developing countries because of its constant requirement of
consumable chemical and consequent higher running costs.
Jar Test it is used to calculate the dosage
of the coagulant.
Advanced chemical processes
Ion exchange- although both natural and
synthetic ion exchange resins are available,
synthetic resins are used more widely
because of their durability. Some natural
zeolites (resins) are also used for the
removal of ammonia from wastewater.
Electrochemical treatment
Wastewater is mixed with seawater and is
passed into a single cell containing carbon
electrodes.
Electro dialysis
Ionic components of a solutions are separated
through the use of semi permeable ion-selective
membranes. If the electrical potential is applied
between the two electrodes, which in turn causes a
migration of cations toward the negative electrode
and migration of anions towards the positive
electrode. Due to the alternate spacing of cation
and anion permeable membranes, cells of
concentrated and dilute salts are formed.
. Principle of simple electrodialysis process. Diagram shows the membrane
configuration with alternating cation-selective (1)and anion-selective (2)
membranes between two electrodes ((3) and (4)), one at each end of the stack.
Oxidation and reduction
Oxidation - Chemical oxidation is used to remove
ammonia, to reduce the concentration of residual
organics and to reduce bacterial and viral contents of
wastewater. At present on of the few process for the
removal of ammonical nitrogen , found operationally
dependable , is chlorination. Ammonia can be removed
chemically by adding chlorine or hypochlorite to form
monochloramine and dichloramine as intermediate
products and nitrogen gas and hydrochloric acid as end
products. Problem associate with this method is the
presence of various organic and inorganic compounds
that will exert chlorine demand. Chemical oxidation of
organic material in wastewater.
Reduction
Nitrates present in wastewater can be reduced
electrolytically or by using strong reducing agents(
e.g. ferrous oxide). The reaction must usually
catalyzed while using reducing agents. The two
step processes using different reducing agents and
catalysts are limited by the availability of
chemicals at low cost, and the fact that the treated
effluent and waste sludge may contain toxic
compounds derived from the chemicals used for
catalyzing various reactions.
Вам также может понравиться
- Mini Refinery Feasibility OverviewДокумент9 страницMini Refinery Feasibility OverviewPouria SabbaghОценок пока нет
- Basic Industrial Wastewater Treatment WorkshopДокумент94 страницыBasic Industrial Wastewater Treatment Workshophenry_tan5328Оценок пока нет
- Wastewater Treatment OverviewДокумент55 страницWastewater Treatment OverviewShorOuq Mohammed Malkawi100% (1)
- LEC 5 Use of Microorganisms in Effluent TreatmentДокумент5 страницLEC 5 Use of Microorganisms in Effluent TreatmentDysmas100% (1)
- Biological Wastewater TreatmentДокумент164 страницыBiological Wastewater TreatmentBenazir Shugufta100% (1)
- Wastewater Treatment: Introduction To BiotechnologyДокумент54 страницыWastewater Treatment: Introduction To Biotechnologyjantskie100% (1)
- Wastewater TreatmentДокумент106 страницWastewater TreatmentFajaryan Wijananto100% (3)
- F CVEN9857 - Biological Nutrient Removal MMДокумент80 страницF CVEN9857 - Biological Nutrient Removal MMWeixuan ZhangОценок пока нет
- Chapter 6 Waste Water TreatmentДокумент93 страницыChapter 6 Waste Water Treatmentshiksha gauliОценок пока нет
- Lecture Iii Cve 503 Biological Treatment of Wastewater1Документ41 страницаLecture Iii Cve 503 Biological Treatment of Wastewater1James Korbla Dodzi Hills100% (1)
- Mixing Mixing: ENVE 301 Environmental Engineering Unit OperationsДокумент47 страницMixing Mixing: ENVE 301 Environmental Engineering Unit OperationsTaha ZiaОценок пока нет
- Lesson 15 - Trickling FiltersДокумент10 страницLesson 15 - Trickling FiltersShane RodriguezОценок пока нет
- What Is Tertiary Treatment PlantДокумент8 страницWhat Is Tertiary Treatment Plantlaloo01Оценок пока нет
- High-Rate Wastewater Treatment by A Shaft-TypeДокумент6 страницHigh-Rate Wastewater Treatment by A Shaft-TypeFabio NascimentoОценок пока нет
- Overview of The Biological TreatmentДокумент4 страницыOverview of The Biological TreatmentJanani SeniappanОценок пока нет
- Unit III Waste Water Treatment and DisposalДокумент26 страницUnit III Waste Water Treatment and DisposalSivaRaman100% (1)
- 9.6.2023 - Shorthand Notes On Sewage Sludge Treatment and DisposalДокумент17 страниц9.6.2023 - Shorthand Notes On Sewage Sludge Treatment and DisposalLucas AgumbaОценок пока нет
- Industrial Wastewater TreatmentДокумент70 страницIndustrial Wastewater TreatmentAudrius100% (3)
- Environmental Engineering (EN) - 20.03.18 PDFДокумент240 страницEnvironmental Engineering (EN) - 20.03.18 PDFRaj VermaОценок пока нет
- Coagulation PDFДокумент72 страницыCoagulation PDFAhmed WagihОценок пока нет
- Active Sludge PDFДокумент8 страницActive Sludge PDFDark_KiroОценок пока нет
- CE-311 Biological Treatment I - Activated Sludge ProcessДокумент40 страницCE-311 Biological Treatment I - Activated Sludge ProcessShubham BansalОценок пока нет
- ENVI Trickling FiltersДокумент23 страницыENVI Trickling FiltersbaBy daBy AnNetTeОценок пока нет
- Wastewater Characterization1Документ39 страницWastewater Characterization1ksbbs100% (1)
- I. Introduction To Air Pollution and Control: Primary PollutantsДокумент26 страницI. Introduction To Air Pollution and Control: Primary PollutantsMoves JaggerОценок пока нет
- Chapter 5Документ23 страницыChapter 5Tefera Temesgen100% (1)
- Settling TanksДокумент33 страницыSettling TanksWei ShienОценок пока нет
- Unit 3Документ155 страницUnit 3rangaОценок пока нет
- STP PDFДокумент16 страницSTP PDFhumaidjafriОценок пока нет
- Water Management1Документ22 страницыWater Management1Angelie Lape100% (1)
- School of Civil Engineering & Architecture: Adama Science and Technology UniversityДокумент112 страницSchool of Civil Engineering & Architecture: Adama Science and Technology UniversityHusen DugoОценок пока нет
- Aeration System in WWTPДокумент10 страницAeration System in WWTPashokОценок пока нет
- Literature Study For Ultra Filtration ProcessДокумент29 страницLiterature Study For Ultra Filtration ProcessSheldonОценок пока нет
- Advanced Wastewater Treatment: Gyeongsang National UniversityДокумент31 страницаAdvanced Wastewater Treatment: Gyeongsang National UniversityEdwin KamalhaОценок пока нет
- 05 SedimentationДокумент70 страниц05 SedimentationAnonymous Cpe6vcОценок пока нет
- CE-311 Biological Treatment I - Trickling FilterДокумент17 страницCE-311 Biological Treatment I - Trickling FilterShubham BansalОценок пока нет
- Activated Sludge Treatment ProcessДокумент7 страницActivated Sludge Treatment ProcessMaizan Sofia100% (1)
- Isea - Wastewater Treatment Plant For Residental Communities and Industrial FacilitiesДокумент19 страницIsea - Wastewater Treatment Plant For Residental Communities and Industrial FacilitiesAG-Metal /Tretman Otpadnih Voda/Wastewater Treatment100% (3)
- Wastewater Treatment Methods Physical Unit Operations Chemical Unit Operations Biological Unit OperationsДокумент34 страницыWastewater Treatment Methods Physical Unit Operations Chemical Unit Operations Biological Unit OperationsNumanОценок пока нет
- LECTURE 11 - Conventional Water Treatment Facility2014Документ51 страницаLECTURE 11 - Conventional Water Treatment Facility2014Kaaviyan thirunyanamОценок пока нет
- Rotating Biological ContactorДокумент6 страницRotating Biological ContactorDebasish NayakОценок пока нет
- Glossary For Water Treatment Systems 2007Документ10 страницGlossary For Water Treatment Systems 2007Ahmad Mamdouh100% (1)
- Nitrification and DenitrificationДокумент4 страницыNitrification and DenitrificationJpricarioОценок пока нет
- CH 9 - Attached Growth ProcessДокумент30 страницCH 9 - Attached Growth Processxuantra92100% (1)
- Activated Sludge - Troubleshooting GuideДокумент5 страницActivated Sludge - Troubleshooting Guidekuthappady0% (1)
- Tertiary TreatmentДокумент45 страницTertiary TreatmentsunitaudayОценок пока нет
- FiltrationДокумент23 страницыFiltrationNeha PatelОценок пока нет
- Course Material Week 10 Biological Process Technology Environmental Engineering Dept. Institut Teknologi BandungДокумент50 страницCourse Material Week 10 Biological Process Technology Environmental Engineering Dept. Institut Teknologi BandungAnnisa MaulinaОценок пока нет
- Coagulation FlocculationДокумент71 страницаCoagulation FlocculationDeepa Singh100% (1)
- 2021 CH 274Документ23 страницы2021 CH 274Ali Raza MeharОценок пока нет
- Primary Treatment of Sewage 3Документ17 страницPrimary Treatment of Sewage 3sasisОценок пока нет
- LamellaДокумент5 страницLamellaKhoa Nguyen DangОценок пока нет
- Types of Screens: Primary TreatmentДокумент20 страницTypes of Screens: Primary TreatmentMadhuri GuptaОценок пока нет
- L13-15 Water+Treatment Coagulation,+Flocculation,+Sedimentation,+FiltrationДокумент13 страницL13-15 Water+Treatment Coagulation,+Flocculation,+Sedimentation,+Filtrationsalil dubey100% (1)
- "Water Treatment Process": (Subject Code: 3714802)Документ33 страницы"Water Treatment Process": (Subject Code: 3714802)mritunjay yadavОценок пока нет
- CalculationДокумент28 страницCalculationManish Kumar Ghora100% (1)
- Chapter-14 Final Aerobic-DigestionДокумент18 страницChapter-14 Final Aerobic-DigestionIrvin joseОценок пока нет
- Wastewater Treatment Mechanic: Passbooks Study GuideОт EverandWastewater Treatment Mechanic: Passbooks Study GuideОценок пока нет
- DAO 1992-29 - IRR of RA6969Документ27 страницDAO 1992-29 - IRR of RA6969Pacific Spectrum100% (1)
- Motorstar R155Документ4 страницыMotorstar R155VIRGILIO MANANGANОценок пока нет
- Waste ManagementДокумент34 страницыWaste ManagementOvertson SyiemliehОценок пока нет
- Law and Logic (Fallacies)Документ10 страницLaw and Logic (Fallacies)Arbie Dela TorreОценок пока нет
- Backyard CattleДокумент9 страницBackyard CattleToy GolosinoОценок пока нет
- RA6969Документ8 страницRA6969Gino Luna CondecionОценок пока нет
- GP For The Rama Phosphates LTD.: Case Study of Soy Oil Processing Industry (DOC Production)Документ39 страницGP For The Rama Phosphates LTD.: Case Study of Soy Oil Processing Industry (DOC Production)VIRGILIO MANANGANОценок пока нет
- Requirements For Building Permit in The PhilippinesДокумент1 страницаRequirements For Building Permit in The PhilippinesVIRGILIO MANANGANОценок пока нет
- Do You Want To Go Into BusinessДокумент50 страницDo You Want To Go Into BusinessDomingo NacionОценок пока нет
- 8hr Training For Managing Heads - (Bellevue, Sept19) General InvitationДокумент4 страницы8hr Training For Managing Heads - (Bellevue, Sept19) General InvitationVIRGILIO MANANGANОценок пока нет
- Design of Concrete Foundation Fr6mt Test BenchДокумент1 страницаDesign of Concrete Foundation Fr6mt Test BenchVIRGILIO MANANGANОценок пока нет
- ISO14001 Implementation & Operation, Checking and Management ReviewДокумент45 страницISO14001 Implementation & Operation, Checking and Management ReviewVIRGILIO MANANGANОценок пока нет
- Safety ProgramДокумент23 страницыSafety ProgramVIRGILIO MANANGANОценок пока нет
- Excel Loan CalculatorДокумент1 страницаExcel Loan CalculatorAbhishek ShrivastavОценок пока нет
- ISO Orientation12Документ24 страницыISO Orientation12VIRGILIO MANANGANОценок пока нет
- Building Fire SafetyДокумент78 страницBuilding Fire SafetyVIRGILIO MANANGANОценок пока нет
- SY Vs CAДокумент5 страницSY Vs CAAyries AntonioОценок пока нет
- ISO Orientation20Документ18 страницISO Orientation20VIRGILIO MANANGANОценок пока нет
- Project Experiences - Gunawan MuktiwibowoДокумент29 страницProject Experiences - Gunawan MuktiwibowoGunawan Raharjo100% (1)
- Harrison Catalog OnlineДокумент20 страницHarrison Catalog OnlineHAROLDОценок пока нет
- Chemical Cleaning ProcessДокумент7 страницChemical Cleaning ProcessmechanikyОценок пока нет
- Conversion of Sulfinol To BASF' S Amdea: SM ® SM SMДокумент12 страницConversion of Sulfinol To BASF' S Amdea: SM ® SM SMvaratharajan g rОценок пока нет
- Compressed Air FiltersДокумент8 страницCompressed Air FiltersJuan Carlos Vazquez RosasОценок пока нет
- Operation Manual For PX ABS 3.4m3 Portable Assembly Biogas SystemДокумент10 страницOperation Manual For PX ABS 3.4m3 Portable Assembly Biogas SystemShahabas ShabuОценок пока нет
- Chemistry Solutions DPP EtoosДокумент8 страницChemistry Solutions DPP EtoosabhishekОценок пока нет
- SPT Cynara Co2 BrochureДокумент2 страницыSPT Cynara Co2 BrochurexaxaxОценок пока нет
- FPFF 1Документ8 страницFPFF 1ShashankNageshОценок пока нет
- The Effects of Hydrogen Fluoride On The Wooden Surface of Historic Buildings During Fire Suppression Using Fluorinated Chemical GasesДокумент12 страницThe Effects of Hydrogen Fluoride On The Wooden Surface of Historic Buildings During Fire Suppression Using Fluorinated Chemical GasesTatiana FabianОценок пока нет
- Notes: Process Flow Diagram Melamine Off-Gas Condensation (Section 25)Документ1 страницаNotes: Process Flow Diagram Melamine Off-Gas Condensation (Section 25)rajindo1Оценок пока нет
- RefrigerantsДокумент149 страницRefrigerantsSatyendraОценок пока нет
- Ashish Shrivastava-Training Consultant ProfileДокумент6 страницAshish Shrivastava-Training Consultant Profileashish shrivastavОценок пока нет
- Simulation of Air Liquefaction Using Aspen PlusДокумент39 страницSimulation of Air Liquefaction Using Aspen PlusAhmed AliОценок пока нет
- Acid DewpointДокумент2 страницыAcid Dewpointachmadh_2010Оценок пока нет
- 110-RB-701 110-VV-703 110-VV-702 110-EE-702 110-EE-703: LegendДокумент1 страница110-RB-701 110-VV-703 110-VV-702 110-EE-702 110-EE-703: LegendDIPANKAR LALAОценок пока нет
- How Much Coal Required To Produce 1 MW Power?: What Is The TPH of A Boiler?Документ5 страницHow Much Coal Required To Produce 1 MW Power?: What Is The TPH of A Boiler?coconut borneoОценок пока нет
- Gas Flaring Basic InformationДокумент4 страницыGas Flaring Basic InformationFriday IjokgwungОценок пока нет
- Pensky MartensДокумент2 страницыPensky Martensharry_chemОценок пока нет
- Flue Gas DesulfurisationДокумент22 страницыFlue Gas DesulfurisationManishankar PandaОценок пока нет
- Questions of CEM PDFДокумент2 страницыQuestions of CEM PDFAnonymous Anzt6fAeM100% (1)
- 氫氣高壓用閥Документ2 страницы氫氣高壓用閥William ChangОценок пока нет
- PE 311 (13 Credits) :: Unit Operation For Petroleum IndustryДокумент52 страницыPE 311 (13 Credits) :: Unit Operation For Petroleum IndustryMbarouk Shaame MbaroukОценок пока нет
- HF Alkylation and NExOCTANE Tech For Gasoline ProductionДокумент42 страницыHF Alkylation and NExOCTANE Tech For Gasoline ProductionUsama Shakil0% (1)
- Modeling and Simulation of Steam CrackersДокумент6 страницModeling and Simulation of Steam CrackersFathan FathullahОценок пока нет
- DHDT BaiscsДокумент2 страницыDHDT BaiscsAvik Bhai100% (1)
- Kinetics of Methanol Synthesis From Carbon Dioxide-Portha2017Документ24 страницыKinetics of Methanol Synthesis From Carbon Dioxide-Portha2017fkgui2014Оценок пока нет
- 021 2008 Abstracts Chemreactor 18 MaltaДокумент558 страниц021 2008 Abstracts Chemreactor 18 MaltaChau MaiОценок пока нет
- Improved Procedure For The Preparation of 1 - (2-Phenethyl) - 4-PiperidoneДокумент5 страницImproved Procedure For The Preparation of 1 - (2-Phenethyl) - 4-PiperidonejesusОценок пока нет