Академический Документы
Профессиональный Документы
Культура Документы
Care of Patients Undergoing Valvular Heart Surgerry
Загружено:
anusarannyaАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Care of Patients Undergoing Valvular Heart Surgerry
Загружено:
anusarannyaАвторское право:
Доступные форматы
CARE OF PATIENTS
UNDERGOING
VALVULAR HEART
SURGERRY
PRESENTED BY:
MS.ANU SARANNYA
M.SC(N) II YEAR
ACON
INTRODUCTION
When heart valves fail to open and close
properly, the implications for the heart can
be serious, possibly hampering the heart's
ability to pump blood adequately through
the body.
TERMINOLOGIES
COAPTATION:
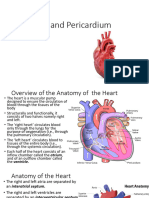
ANATOMY AND PHYSIOLOGY OF
VALVES
Valves are actually flaps (leaflets) that act
as one-way inlets for blood coming into a
ventricle and one-way outlets for blood
leaving a ventricle.
Normal valves have three flaps (leaflets),
except the mitral valve, which only has two
flaps
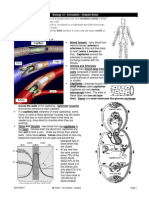
VALVES OF THE HEART
Tricuspid valve. This valve is located
between the right atrium and the right
ventricle.
Pulmonary valve. The pulmonary valve is
located between the right ventricle and the
pulmonary artery.
Mitral valve. This valve is located between
the left atrium and the left ventricle.
Aortic valve. The aortic valve is located
between the left ventricle and the aorta.
ATRIO VENTRICULAR VALVES
Mitral valve
Tricuspid valve
SEMILUNAR VALVES
Aortic valve
Pulmonary valve
ATRIOVENTRICULAR VALVE
These are the mitral and tricuspid valves
situated between the atria and the ventricles that
prevent backflow from the ventricles into the
atria during systole.
They are anchored to the walls of the ventricles
by chordae tendineae, which prevent the valves
from inverting.
ATRIOVENTRICULAR VALVE
The chordae tendineae are attached to
papillary muscles that cause tension to
better hold the valve.
Together, the papillary muscles and the
chordae tendineae are known as the
subvalvular apparatus
opening and closure of the valves IS
entirely by the pressure gradient across
the valve
ATRIOVENTRICULAR VALVE
The mitral valve is also called the bicuspid
valve because it contains two leaflets or
cusps.
The mitral valve gets its name from the
resemblance to a bishop's mitre (a type of
hat).
It is on the left side of the heart and allows
the blood to flow from the left atrium into
the left ventricle.
ATRIOVENTRICULAR VALVE
The closure of the AV valves is heard as
lub, the first heart sound (S1). The closure
of the SL valves is heard as dub, the
second heart sound (S2).
SEMILUNAR VALVES
The semilunar valves, the aortic and the
pulmonary valves, are located at the base
of the aorta and the pulmonary trunk or
artery, and the aorta.
These two arteries receive blood from the
ventricles and their semilunar valves
permit blood to be forced into the arteries,
and prevent backflow from the arteries into
the ventricles
SEMILUNAR VALVES
The valve opens in ventricular systole,
when the pressure in the ventricle
rises above the pressure in the
artery. At the end of ventricular
systole, when the pressure in the
ventricle falls rapidly, the pressure in
the artery will close the pulmonary
valve.
SEMILUNAR VALVES
The closure of the aortic valve contributes
the A2 component of the second heart
sound (S2).
The closure of the pulmonary valve
contributes the P2 component of the
second heart sound (S2)
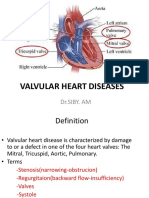
HEART VALVE DISEASE
Valvular heart disease is any disease process
involving one or more of the four valves of the
heart (the aortic and mitral valves on the left and
the pulmonary and tricuspid valves on the right).
Collectively and anatomically, the valves are part
of the dense connective tissue makeup of the
heart known as the cardiac skeleton.
Valve problems may be congenital (inborn) or
acquired (due to another cause later in life).
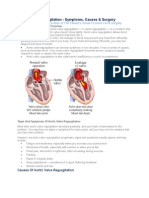
REGURGITATION
This means the valve doesn't close
completely, causing the blood to flow
backward through the valve.
This results in leakage of blood back into
the atria from the ventricles (in the case of
the mitral and tricuspid valves) or leakage
of blood back into the ventricles (in the
case of the aortic and pulmonary valves).
STENOSIS
The valve opening is narrowed and the
valve doesn't open properly, inhibiting the
ability of the heart to pump blood across
the narrowed valve due to the increased
force required to pump blood through the
stiff (stenotic) valve(s).
ATRESIA
This means the valve opening doesn't
develop at all, preventing blood from
passing from an atria to a ventricle, or
from a ventricle to the pulmonary artery or
aorta. Blood must find an alternate route,
usually through another existing congenital
(present at birth) defect, such as an atrial
septal defect or a ventricular septal defect.
Valve involved Stenotic disease Insufficiency/regurgitation
disease
Aortic valve Aortic valve stenosis Aortic
insufficiency/regurgitation
Mitral valve Mitral valve stenosis Mitral
insufficiency/regurgitation
Tricuspid valve Tricuspid valve
stenosis
Tricuspid
insufficiency/regurgitation
Pulmonary valve Pulmonary valve
stenosis
Pulmonary
insufficiency/regurgitation
Aortic and mitral valve disorders,These
are termed left heart diseases.
Pulmonary and tricuspid valve disorders
are right heart diseases. Pulmonary valve
diseases are the least common heart
valve disease in adults.
DYSPLASIA
Heart valve dysplasia is an error in the
development of any of the heart valves,
and a common cause of congenital heart
defects in humans as well as animals;
tetralogy of Fallot is a congenital heart
defect with four abnormalities, one of
which is stenosis of the pulmonary valve.
Ebstein's anomaly is an abnormality of the
tricuspid valve
RHEUMATIC HEART DISEASE
Valvular heart disease resulting from
rheumatic fever is referred to as
"rheumatic heart disease".
SURGICAL
MANAGEMENT
MITRAL VALVE ANNULOPLASTY
Mitral regurgitation is the most common
form of mitral valve dysfunction. Today
more than 2.5 million Americans are
estimated to be affected by mitral
regurgitation.
This number is expected to double by the
year 2030. Every year, 300,000 people
worldwide undergo open heart surgery for
mitral valve repair, 44,000 people in the
US alone
GOAL
The goal of mitral valve annuloplasty is to
regain mitral valve competence by restoring
the physiological form and function of the
normal mitral valve apparatus.
SEMI RIGID RINGS
The goal of semi-rigid rings is to maintain
coaptation and valve integrity during
systole, while allowing for good
hemodynamics during diastole. Rigid rings
are designed to provide rigid support in
large dilation and under high-pressure.
Annuloplasty devices are classified by the
U.S. Food and Drug Administration (FDA)
as class II medical device. According to
the FDA following issues need to be
addressed before annuloplasty rings can
be approved for marketing:
Biocompatibility testing
Computational structural analysis
Tensile testing
Suture pull-out testing
Sterilization validation
Biological testing including bioburden and
pyrogen testing
Shelf-life validation
MINIMALLY INVASIVE
ANNULOPLASTY
New technologies that allow for minimally
invasive surgery have also begun to
change mitral valve surgery. A number of
devices and techniques have been
developed that do not necessitate open-
heart surgery for the repair of the mitral
valve
BALLOON MITRAL VALVOTOMY
Balloon valvotomy
successfully opens
the narrowed valve
and improves the
overall function of the
heart
INDICATIONS
select patients who have mitral valve
stenosis with symptoms
older patients who have aortic valve
stenosis, but are not able to undergo
surgery
some patients with pulmonic valve
stenosis
PROCEDURE
Long, slender tubes called catheters are
first placed into blood vessels in the groin
and guided into the chambers of the heart
The catheter is positioned so the balloon
tip is directly inside the narrowed valve
When the opening of the valve has been
widened enough, the balloon is deflated
and removed.
COMMISUROTOMY
Fused valve leaflets, or flaps, are
separated to widen the valve opening.
A median sternotomy is generally used,
but a right antero-lateral thoracotomy
through a submammary incision is an
excellent optional approach for mitral
commissurotomy and may be preferred for
cosmetic reasons
If there is localised calcification
of the commissures,
debridement with a rongeur is
carefully performed first,
avoiding injury of the leaflets
Often, the commissural
chordae are thickened and
shortened and the papillary
muscle may even come right to
the leaflet. When this happens,
the head of the muscle is
incised longitudinally to permit
a wide separation of the
leaflets
One of the advantages of the open
commissurotomy is the possibility of
immediate assessment of the valve
anatomy and function. This is usually done
by pressurising the left ventricle with saline
injected through the mitral valve with a
bulb syringe
Method of testing by
pumping saline
through an apical LV
vent cannula. Air is
allowed to escape
through an aortic root
vent.
VALVE REPLACEMENT
Mechanical -- made of man-made
materials, such as titanium and carbon.
These valves last the longest. You will
need to take blood-thinning medicine,
such as warfarin (Coumadin) or aspirin, for
the rest of your life.
Biological -- made of human or animal
tissue. These valves last 10 - 12 years, but
you may not need to take blood thinners
for life
BIOPROSTHETIC
VALVE
MECHANICAL
VALVE
VALVE REPLACEMENT SURGERY
Valve replacement surgery is the
replacement of one or more of the heart
valves with either an artificial heart valve
or a bioprosthesis (homograft from human
tissue or xenograft e.g. from pig). It is an
alternative to valve repair
PROCEDURES
Aortic valve replacement
Mitral valve replacement
Tricuspid valve replacement
Pulmonary valve replacement
AORTIC VALVE REPLACEMENT
Aortic valve replacement is a procedure in
which a patient's failing aortic valve is
replaced with an artificial heart valve. The
aortic valve can be affected by a range of
diseases; the valve can either become
leaky (aortic insufficiency / regurgitation)
or partially blocked (aortic stenosis).
Current aortic valve replacement
approaches include
open heart surgery,
minimally invasive cardiac surgery (MICS)
minimally invasive, catheter-based
(percutaneous) aortic valve replacement.
TISSUE VALVES
Tissue heart valves are usually made from
animal tissue, either animal heart valve
tissue or animal pericardial tissue. The
tissue is treated to prevent rejection and
calcification
homograft - a human aortic valvecan be
implanted.
Another procedure for aortic valve replacement
is the Ross procedure (or pulmonary autograft).
In a Ross procedure, the aortic valve is removed
and replaced with the patient's own pulmonary
valve. A pulmonary homograft (pulmonary valve
taken from a cadaver) is then used to replace
the patient's own pulmonary valve. This
procedure was first used in 1967 and is used
primarily in children, as the procedure allows the
patient's own pulmonary valve (now in the aortic
position) to grow with the child.
ROSS PROCEDURE
The Ross Procedure is a type of
specialized aortic valve surgery where the
patient's diseased aortic valve is replaced
with his or her own pulmonary valve. The
pulmonary valve is then replaced with
cryopreserved cadaveric pulmonary valve.
In children and young adults, or older
particularly active patients, this procedure
offers several advantages over traditional
aortic valve replacement with
manufactured prostheses
The pulmonary valve and a segment of the pulmonary
artery are excised. This pulmonary segment will later be
placed in the aortic position replacing the diseased aortic
valve.
The diseased aortic valve and proximal tissue is
removed, leaving the right and left coronary arteries
with only a button of tissue.
The pulmonary autograft is placed in the aortic position and the
buttons of tissue on both the right and left coronary arteries are
then sewn into that pulmonary segment and closed. A cadaveric
pulmonary valve and artery homograft is then replaced in the
pulmonary position to replace the excised pulmonary segment.
PROCEDURE
Aortic valve replacement is most frequently done
through a median sternotomy, meaning the
incision is made by cutting through the sternum.
Once the pericardium has been opened, the
patient is put on a cardiopulmonary bypass
machine, also known as the heart-lung machine.
This machine takes over the task of breathing for
the patient and pumping their blood around
while the surgeon replaces the heart valve.
Once the patient is on bypass, a cut is made in
the aorta and a crossclamp applied.
The surgeon then removes the patient's diseased aortic
valve and a mechanical or tissue valve is put in its place.
Once the valve is in place and the aorta has been
closed, the patient is taken off the heart-lung machine.
Transesophageal echocardiogram (TEE, an ultra-sound
of the heart done through the esophagus) can be used to
verify that the new valve is functioning properly.
Pacing wires are usually put in place, so that the heart
can be manually paced should any complications arise
after surgery.
Drainage tubes are also inserted to drain fluids from the
chest and pericardium following surgery. These are
usually removed within 36 hours while the pacing wires
are generally left in place until right before the patient is
discharged from the hospital
MITRAL VALVE REPLACEMENT
Mitral valve replacement is a cardiac surgical
procedure in which a patients diseased mitral
valve is replaced by either a mechanical or
bioprosthetic valve. Mitral valve replacement is
performed when the valve becomes too tight
(mitral valve stenosis) for blood to flow into the
left ventricle, or too loose (mitral valve
regurgitation) in which case blood can leak back
into the left atrium and thereby back into the
lung. Mitral valve disease can occur from
infection, calcification, inherited collagen
disease, or other causes
SURGICAL PROCEDURE
Patients having mitral valve surgery receive general
anesthesia.
Incision can be made somewhat horizontally under the
left breast, or vertically through the sternum.
After the heart is exposed, canulae are placed to rerout
blood to a heart-lung machine for cardiopulmonary
bypass.
An incision is made in the left atrium to expose the mitral
valve.
The valve is then replaced with either a biological or
mechanical valve. The left artium is then closed, and the
patient weaned from cardiopulmonary bypass. After
surgery patients are typically taken to an intensive care
unit (ICU).
TRICUSPID VALVE REPLACEMENT
The tricuspid valve is often considered in the workup of
heart failure only after more prominent cardiac
pathologies such as aortic, mitral, and coronary
atherosclerotic disease have been discussed, and as
such it has been referred to as the forgotten valve.
The sequelae of significant tricuspid regurgitation can be
significant however and include ascites,
hepatosplenomegaly, pleural effusions, and peripheral
edema.
Tricuspid regurgitation is usually secondary to left-sided
valvular pathology (commonly the mitral valve) causing
elevated pulmonary pressures with subsequent dilation
of the tricuspid annulus.
Rheumatic disease, Ebstein's anomaly, and endocarditis
are other important causes of tricuspid incompetence
SURGICAL PROCEDURE
Aortic and bi-caval cannulation is
accomplished with direct cannulation of
the superior vena cava (SVC) and inferior
vena cava (IVC).
Caval tapes are snared around the IVC
and SVC to achieve right heart isolation
A caval clamp can be used alternatively in
the setting of significant adhesions. An
oblique right atriotomy is performed down
to the IVC cannula, incorporating any
exisiting retrograde catheter site
A self retaining retractor is used.
Occasionally pledgeted traction sutures
are placed on the edges of the atriotomy
to enhance exposure
The tricuspid valve is inspected and an
assessment of repair versus replacement
is made.
When repair is not feasible, our preference
is to use a bioprosthetic bovine pericardial
valve.
The leaflets are left in place to preserve
the sub-valvular apparatus.
When prolapsing leaflets are large and
bulky, they are fenestrated along a radial
axis, which allows them to fold out of the
way while preserving the tissue.
Everting 2-0 pledgeted Ticron sutures are
placed along the circumference of the
annulus from the atrial to the ventricular
side of the valve, starting at the anterior
leaflet and working clockwise. Great care
is taken when suturing near the AV node
along the septal leaflet
The AV node lies in the triangle of Koch
bordered by the tendon of Todaro, the
septal leaflet of the tricuspid valve and the
orifice of the coronary sinus. Careful
suture placement in this region is
mandatory to avoid injury to the
conduction system
The pulmonary artery catheter is replaced
through the valve and confirmed in
position in the pulmonary artery by
palpation.
The atriotomy is closed with 4-0
polypropylene sutures in two layers after
de-airing maneuvers. The caval tapes are
released and the patient is weaned from
cardiopulmonary bypass.
CHORDAL TRANSFER FOR REPAIR OF
ANTERIOR LEAFLET PROLAPSE
Chordal transfer is a reliable technique for
correction of anterior leaflet prolapse. In most
cases, normal chordae and a strip of leaflet
tissue are transferred from the posterior leaflet to
the free edge of unsupported anterior leaflet; the
posterior leaflet is repaired as after a
quadrangular resection with either a sliding
repair or a standard repair.
Occasionally, secondary anterior leaflet chordae
may be transferred from the undersurface of the
anterior leaflet to its unsupported free edge,
effecting a rapid and effective repair. After
chordal transfer, annuloplasty completes the
mitral valve repair.
Вам также может понравиться
- Valve SurgeriesДокумент13 страницValve SurgeriesShreeja SajitОценок пока нет
- Valve Repair & ReplacementДокумент27 страницValve Repair & ReplacementmaibejoseОценок пока нет
- Valvular Heart Defects and Infective Endocarditis-3Документ37 страницValvular Heart Defects and Infective Endocarditis-3nicholasacquah680Оценок пока нет
- Biofluid Assignment 3Документ6 страницBiofluid Assignment 3Dina AssefaОценок пока нет
- The Heart 16th March 13Документ105 страницThe Heart 16th March 13Asmara SyedОценок пока нет
- Anatomy and Physiology of The Valves of The Heart: 1) Tricuspid ValveДокумент17 страницAnatomy and Physiology of The Valves of The Heart: 1) Tricuspid ValveIvan YeohОценок пока нет
- Cardiac Anatomy and PhysiologyДокумент57 страницCardiac Anatomy and PhysiologyRheecha JoshiОценок пока нет
- Aortic ValveДокумент8 страницAortic ValveIfeanyichukwu OgbonnayaОценок пока нет
- Biomaterials Ppt-Part - 2 - CompressedДокумент176 страницBiomaterials Ppt-Part - 2 - Compressedsparsh agarwalОценок пока нет
- Riya Arya - 21msc1279 - Biology For ChemistsДокумент13 страницRiya Arya - 21msc1279 - Biology For ChemistsSwadesh SenОценок пока нет
- Heart SurgeriesДокумент16 страницHeart SurgeriesPrincess Lee DangilanОценок пока нет
- Cardiovascular System - The HeartДокумент77 страницCardiovascular System - The HeartZiraili VidalОценок пока нет
- Cardiac AnatomyДокумент46 страницCardiac AnatomyRheechaОценок пока нет
- Valvular Disorders: Reported By: Charlene Dorothy S. TabigneДокумент34 страницыValvular Disorders: Reported By: Charlene Dorothy S. TabigneCharlene TabigneОценок пока нет
- Echocardiographic Evaluation of Aortic ValveДокумент89 страницEchocardiographic Evaluation of Aortic ValveSruthiОценок пока нет
- Common Heart Valve DisordersДокумент7 страницCommon Heart Valve DisordersJudy LeeОценок пока нет
- The Human Heart: Dr. Nwosu C.I.A., MSC, MBBS, MDДокумент38 страницThe Human Heart: Dr. Nwosu C.I.A., MSC, MBBS, MDcoolblue89Оценок пока нет
- Mitral StenosisДокумент43 страницыMitral StenosisEricka SantosОценок пока нет
- Valves of The HeartДокумент10 страницValves of The Heartjishnunarayanan2003Оценок пока нет
- DFF FFF AaaaaДокумент5 страницDFF FFF AaaaaffffffffОценок пока нет
- Circulatory SystemДокумент17 страницCirculatory Systemmarissa quijanoОценок пока нет
- Week 11 THE HEART 2021Документ60 страницWeek 11 THE HEART 2021Huffy PotterОценок пока нет
- Valvular Heart DefectsДокумент26 страницValvular Heart DefectsHarold DiasanaОценок пока нет
- MURMURДокумент21 страницаMURMURtoyyibОценок пока нет
- VALVULAR HEART DISEASE Copy AutosavedДокумент33 страницыVALVULAR HEART DISEASE Copy AutosavedShienna Rose Ann ManaloОценок пока нет
- WK 1& 2 LectureДокумент149 страницWK 1& 2 LectureElma SekikorolevuОценок пока нет
- HeartДокумент23 страницыHeartRonald A. OganiaОценок пока нет
- Complex Functional Single VentricleДокумент35 страницComplex Functional Single Ventriclethecount28991Оценок пока нет
- Cardiovascular SystemДокумент53 страницыCardiovascular SystemPelin OktayОценок пока нет
- A. Cardio: Anatomy & Physiology - A. AnatomyДокумент43 страницыA. Cardio: Anatomy & Physiology - A. AnatomyGloryJaneОценок пока нет
- Caso Clinico Cambio de Valvula AorticaДокумент11 страницCaso Clinico Cambio de Valvula Aorticaapi-670089280Оценок пока нет
- Capillary: Nutrients To, and Take Wastes Away From, CellsДокумент7 страницCapillary: Nutrients To, and Take Wastes Away From, CellseyhethОценок пока нет
- Tetralogy of FallotДокумент28 страницTetralogy of FallotLaurice Grace Zamayla BaelОценок пока нет
- VHD: Types, Causes, Symptoms & ManagementДокумент42 страницыVHD: Types, Causes, Symptoms & ManagementareeparambilОценок пока нет
- Chapter 7. CirculationДокумент54 страницыChapter 7. CirculationankurbiologyОценок пока нет
- CVS up to cardiac output-1Документ198 страницCVS up to cardiac output-1mokshachapaneriОценок пока нет
- Structure & Function of the HeartДокумент18 страницStructure & Function of the HeartDeep RoyОценок пока нет
- Intra-Aortic Balloon Pump GuideДокумент27 страницIntra-Aortic Balloon Pump GuideJadie Barringer IIIОценок пока нет
- The Heart ValvesДокумент7 страницThe Heart ValvesJordan MosesОценок пока нет
- Clinical Case 01-09-22Документ9 страницClinical Case 01-09-22api-626421480Оценок пока нет
- IV TherapyДокумент15 страницIV Therapyprincessmonique_208609100% (2)
- Heart Valve SurgeryДокумент16 страницHeart Valve SurgeryMundhir Al-KhusaibiОценок пока нет
- Acquired Heart DXДокумент39 страницAcquired Heart DXFauziana Abd JabarОценок пока нет
- Pathophysiology of Aortic Valve DiseaseДокумент0 страницPathophysiology of Aortic Valve DiseaseIndah Afriani NasutionОценок пока нет
- IGCSE Biology Transport in Animals NotesДокумент62 страницыIGCSE Biology Transport in Animals NotesSir AhmedОценок пока нет
- Aortic Valve Disease General OverlookДокумент2 страницыAortic Valve Disease General OverlookAlex prhОценок пока нет
- Session 5Документ41 страницаSession 5tazebОценок пока нет
- What is a prosthetic heart valveДокумент10 страницWhat is a prosthetic heart valveRenu SamuelОценок пока нет
- Cardiovascular DisordersДокумент94 страницыCardiovascular DisordersLui Andrei AnilaОценок пока нет
- Aortic Valve RegurgitationДокумент3 страницыAortic Valve RegurgitationMarky StrikerОценок пока нет
- Interventionalprocedures 254Документ38 страницInterventionalprocedures 254waqas_xsОценок пока нет
- Circulatory SystemДокумент96 страницCirculatory SystemuzumakiyashОценок пока нет
- Cadiac Cycle, Heart Sound, ECG, HypertensionДокумент110 страницCadiac Cycle, Heart Sound, ECG, HypertensionNilesh100% (1)
- Cardiovascular DiseaseДокумент66 страницCardiovascular DiseaseManohar SinghОценок пока нет
- The Heart and PericardiumДокумент40 страницThe Heart and PericardiumMartha Orendu Oche AttahОценок пока нет
- Lecture #2Документ2 страницыLecture #2yeeticusfinchlmaoОценок пока нет
- Heart Valve SurgeryДокумент4 страницыHeart Valve SurgeryYen NgoОценок пока нет
- CardiovascularsystemДокумент8 страницCardiovascularsystemAkshaya SrinivasanОценок пока нет
- Cardiovascular Patho 1 OgenaДокумент87 страницCardiovascular Patho 1 OgenaQuolette ConstanteОценок пока нет
- Rajan Industrial Safety Assessment - Internship ReportДокумент17 страницRajan Industrial Safety Assessment - Internship ReportanusarannyaОценок пока нет
- Labor Laws and UnionsДокумент149 страницLabor Laws and UnionsanusarannyaОценок пока нет
- Conceptual FrameworkДокумент1 страницаConceptual FrameworkanusarannyaОценок пока нет
- RHDДокумент57 страницRHDanusarannyaОценок пока нет
- Study of X - Ray's in ErДокумент38 страницStudy of X - Ray's in EranusarannyaОценок пока нет
- HAND OUT-haemodynamic MonitoringДокумент1 страницаHAND OUT-haemodynamic MonitoringanusarannyaОценок пока нет
- Coronary Artery Disease: Presented byДокумент34 страницыCoronary Artery Disease: Presented byanusarannyaОценок пока нет
- Nurse As Change AgentДокумент18 страницNurse As Change AgentanusarannyaОценок пока нет
- Ajitha EndocarditisДокумент66 страницAjitha EndocarditisanusarannyaОценок пока нет
- CADДокумент20 страницCADanusarannyaОценок пока нет
- 12-Lead ECG Guide: Limb, Augmented & Chest LeadsДокумент1 страница12-Lead ECG Guide: Limb, Augmented & Chest LeadsanusarannyaОценок пока нет
- Pulmonary Rehab for COPD PatientsДокумент6 страницPulmonary Rehab for COPD PatientsanusarannyaОценок пока нет
- Study of X - Ray's in ErДокумент38 страницStudy of X - Ray's in EranusarannyaОценок пока нет
- Pulmonary Rehab for COPD PatientsДокумент6 страницPulmonary Rehab for COPD PatientsanusarannyaОценок пока нет
- Case Reflection FormatДокумент9 страницCase Reflection Formatanusarannya100% (5)
- Professional Advancement & Role and Scope of NursingДокумент15 страницProfessional Advancement & Role and Scope of Nursinganusarannya100% (5)
- Assignment: Fundamental of ElementsДокумент7 страницAssignment: Fundamental of ElementsanusarannyaОценок пока нет
- Assignment: Fundamental of ElementsДокумент7 страницAssignment: Fundamental of ElementsanusarannyaОценок пока нет
- Nutri ProbДокумент9 страницNutri ProbanusarannyaОценок пока нет
- Presentation Utk State Meetng (DR DAUD)Документ45 страницPresentation Utk State Meetng (DR DAUD)Mohd Daud Che YusofОценок пока нет
- Cardiopulmonary ResuscitationДокумент10 страницCardiopulmonary ResuscitationZacchariah Zeref CaraigОценок пока нет
- Exploring the skills and qualities of effective counselingДокумент12 страницExploring the skills and qualities of effective counselingDinesh Cidoc100% (3)
- Soil AnalysisДокумент12 страницSoil AnalysisSunit Hazarika100% (1)
- Allie Cannon Anxiety Case StudyДокумент9 страницAllie Cannon Anxiety Case Studyapi-349885103Оценок пока нет
- Seronegative Spondyloarthropathies & Inflammatory Low Back Pain - Part 2 - Rayner & Smale PDFДокумент8 страницSeronegative Spondyloarthropathies & Inflammatory Low Back Pain - Part 2 - Rayner & Smale PDFItai IzhakОценок пока нет
- Caring - Watson & SwansonДокумент35 страницCaring - Watson & SwansonDenis ArindaОценок пока нет
- Complicatii Si Sechele Tardive Dupa Tratamentul Multimodal Al GlioamelorДокумент35 страницComplicatii Si Sechele Tardive Dupa Tratamentul Multimodal Al GlioamelorBiblioteca CSNTОценок пока нет
- ADHD Strategies and TipsДокумент26 страницADHD Strategies and Tipsgerfelc03Оценок пока нет
- Safety Data SheetДокумент7 страницSafety Data SheettienОценок пока нет
- Hazardous Waste Generator Online RegistrationДокумент12 страницHazardous Waste Generator Online RegistrationTricia LacuestaОценок пока нет
- Nursing Care Plan Pneumonia With Congenital Heart DiseaseДокумент18 страницNursing Care Plan Pneumonia With Congenital Heart DiseaseKarri Ann Tonel100% (2)
- Abcdefg1983 PDFДокумент769 страницAbcdefg1983 PDFmohОценок пока нет
- Magnesium For LifeДокумент3 страницыMagnesium For Lifeluis alejandro osorioОценок пока нет
- Introduction To Clinical AssessmentДокумент13 страницIntroduction To Clinical AssessmentnurmeenОценок пока нет
- Keneuoe MohlakoanaДокумент137 страницKeneuoe MohlakoanaDurga BhavaniОценок пока нет
- Achilles Tendon ProtocolДокумент6 страницAchilles Tendon Protocolomad pendaftaranPPDSОценок пока нет
- Blue Book - Guidelines For The Control of Infectious DiseaseДокумент271 страницаBlue Book - Guidelines For The Control of Infectious DiseaseavarathunnyОценок пока нет
- Dubai Beauty Salon Nail Technician Joyce Castanos ResumeДокумент1 страницаDubai Beauty Salon Nail Technician Joyce Castanos ResumeAbdul Jakeem CastanosОценок пока нет
- Individual Assignment A1 Engineering Technologist in Society Clb40002Документ13 страницIndividual Assignment A1 Engineering Technologist in Society Clb40002Anonymous T7vjZG4otОценок пока нет
- PICU HandbookДокумент113 страницPICU HandbookCarkos Moreno100% (2)
- ThalassemiaДокумент1 страницаThalassemiaghee91Оценок пока нет
- Water Electrolytes Part Clinical BiochemistryДокумент27 страницWater Electrolytes Part Clinical BiochemistryAshraf FirdadОценок пока нет
- Process Consultation Revisited Building the Helping RelationshipДокумент6 страницProcess Consultation Revisited Building the Helping Relationshipagueviv50% (4)
- Angina PectorisДокумент3 страницыAngina PectorisKhalid Mahmud Arifin100% (2)
- Epididymal CystДокумент6 страницEpididymal CystShree Kumar MishraОценок пока нет
- Emergency RoomДокумент2 страницыEmergency Roomapi-508765756Оценок пока нет
- ANSI/ASHRAE/ASHE Standard 170-2008 Tables for Filter Efficiencies and Design ParametersДокумент4 страницыANSI/ASHRAE/ASHE Standard 170-2008 Tables for Filter Efficiencies and Design ParametersPradeep SukumaranОценок пока нет
- Guide to Selecting a Cooling Water Treatment ProgramДокумент2 страницыGuide to Selecting a Cooling Water Treatment ProgrammoncryОценок пока нет
- Perioperative Venous ThromboembolismProphylaxiДокумент24 страницыPerioperative Venous ThromboembolismProphylaxiElías MendezОценок пока нет