Академический Документы
Профессиональный Документы
Культура Документы
Kinetika Fix
Загружено:
herzi rahmatul s0 оценок0% нашли этот документ полезным (0 голосов)
10 просмотров13 страницОригинальное название
kinetika fix.pptx
Авторское право
© © All Rights Reserved
Доступные форматы
PPTX, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PPTX, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
10 просмотров13 страницKinetika Fix
Загружено:
herzi rahmatul sАвторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PPTX, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 13
Topik 4
Kinetics is important in all phases of the drug
development process as well as in quality
control, stability, bioavailability, and therapeutic
drug monitoring.
Drug kinetics
(pharmacokinetics) describes how the
body handles a drug and accounts for the
processes of absorption, distribution,
metabolism, and elimination.
Drug treatment requires getting a drug to its specific
target site or sites in tissues where the drug performs
its action. Typically, the drug is introduced into the
body (the process of administration), sometimes far
from this target site. The drug must move into the
bloodstream (the process of absorption) and be
transported to the target sites where the drug is
needed (the process of distribution). Some drugs are
chemically altered (the process of metabolism) by the
body before they perform their action, others are
metabolized afterward, and still others are not
metabolized at all. The final step is the removal of the
drug and its metabolites from the body (the process
of elimination).
Many factors, including a persons weight,
genetic makeup, and kidney or liver function,
can influence these kinetic processes
Drug dissolution from dosage forms that do not
disaggregate and release the drug slowly can be
represented by the equation:
Rearrangement of equation (3) yields:
Where Qt is the amount of drug dissolved in time
t, Q0 is the initial amount of drug in the solution
(most times, Q0 = 0) and K0 is the zero order
release constant expressed in units of
concentration/time.
(Dash, Suvakanta, 2010)
To study the release kinetics, data obtained from
in vitro drug release studies were plotted as
cumulative amount of drug released versus time.
Application: This relationship can be used to
describe the drug dissolution of several types of
modified release pharmaceutical dosage forms,
as in the case of some transdermal systems, as
well as matrix tablets with low soluble drugs in
coated forms, osmotic systems, etc.
(Dash, Suvakanta, 2010)
The term k is the zero-order
rate constant and is expressed
in units of mass/time (eg,
mg/min).
Integration
of
Equation 2.19 yields the
following expression:
where A 0 is the amount of drug
at t = 0. Based on this
expression , a graph of A
versus t yields a straight line .
The y intercept is equal to A 0,
and the slope of the line is equal
to k 0.
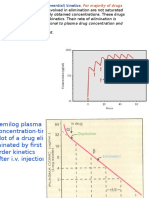
First orde reaction
pharmacetic reactions that often
happen such as: drug absorption
and degradation of the drug
Speed of reaction is influenced by
the concentration of the reactants
d C
kc
dt
t
dC
c0 c k 0 dt
ln c ln c0 k (t 0)
c
ln c ln c0 kt
log c log c0 kt
Martin, 1993
2,303
Half life (t1/2)
The time it takes to decay/loss of substance,
became half.
For a first-order:
0,693
t1 / 2
k
Martin, 1993
Time Expired (t90)
Time a substance has been broken down to live
to 90% of the initial concentration (i.e., breaks
down 10%).
For a first-order:
0,105
t90
k
Martin, 1993
konsentrasi
Log konsentrasi
Plot the concentration and log
concentration against time
waktu
Kemiringan
2,303/k
waktu
Martin, 1993
Differences between zero-order kinetics
and Fist order kinetics
(Rao,Koteswara,G.S.N)
(Rao,Koteswara,G.S.N)
Вам также может понравиться
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Valproic AcidДокумент19 страницValproic Acidherzi rahmatul sОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5795)
- Lidoka inДокумент17 страницLidoka inherzi rahmatul sОценок пока нет
- AparatusДокумент15 страницAparatusherzi rahmatul sОценок пока нет
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- Medical PresipitationДокумент16 страницMedical Presipitationherzi rahmatul sОценок пока нет
- DosisДокумент8 страницDosisherzi rahmatul sОценок пока нет
- Orde 0Документ6 страницOrde 0herzi rahmatul sОценок пока нет
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Directly Proportional To Plasma Drug Concentration and Their Clearance Remains ConstantДокумент6 страницDirectly Proportional To Plasma Drug Concentration and Their Clearance Remains Constantherzi rahmatul sОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Contact AngleДокумент33 страницыContact Angleherzi rahmatul sОценок пока нет
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)