Академический Документы
Профессиональный Документы
Культура Документы
Chem Project
Загружено:
api-3053310330 оценок0% нашли этот документ полезным (0 голосов)
30 просмотров10 страницОригинальное название
chem project
Авторское право
© © All Rights Reserved
Доступные форматы
PPTX, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PPTX, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
30 просмотров10 страницChem Project
Загружено:
api-305331033Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PPTX, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 10
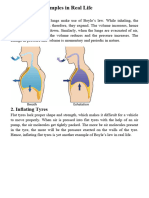
GAS LAW PROJECT
By: Olivia Kaminski
History Info:
◦ A hot air balloon is a balloon used for traveling through the air in a basket that is suspended
below a massive bag of air that is heated
◦ Balloons work by gravity and also the hot air weighs less than cold air does
◦ These balloons are traveling by wind and air essential
◦ Many people enjoy going on rides in them and also for long distance traveling
How it Works:
◦ The process behind why balloons rise is because
warmer air rises in cooler air. This is because hot air is
lighter than cool air as it has less mass per unit of
volume.
◦ Mass can be defined by the measure of how much
matter something contains. The actual balloon has to be
so large as it takes such a large amount of heated air to
lift it off the ground.
◦ To help keep the balloon in the air and rising, hot air
needs to be propelled upwards into the envelope using
the burner
How it Works:
Gas Law:
◦ Charles’s Law states that the volume of a gas is directly related to the
temperature of that certain gas
-In this case it is when the gas is heated from the hot air
balloons burner
◦ This law states that if a given quantity of gas is held at a constant
pressure, its volume is directly proportional to the absolute
temperature
◦ When the gas is heated the balloon starts to expand, it becomes less
dense and provides the balloon to lift off the ground
◦ Hot air is lighter than cool air because it has less mass of the volume
◦ To keep the balloon up in the air the burner that heats the air inside
of the balloon is using propane
How it Works:
◦ Define: Aerosol is really a cloud of liquid and gas that comes out of an aerosol can, not the can itself. In fact,
to be strictly correct about it, an aerosol is a fine mist of liquid, or lots of solid particles, widely and evenly
dispersed throughout a gas.
◦ On the back of an aerosol can it says "pressurized container" and "contents stored under pressure." This is
there so the paint comes out right
◦ When you press the button on the top of an aerosol can, the manufacturers have to squeeze the contents
inside with a compressor
◦ Typically, the contents of an aerosol are stored at 2–8 times normal atmospheric pressure. That's why
aerosols really rush out when you press their buttons.
Continued:
◦ Aerosol cans contain two different substances:
-liquid product that you want to come out
-Pressurized gas called a propellant
◦ This helps to push the liquid product into the air and turn it into an aerosol cloud.
◦ The propellant gas usually turns into a liquid when it's forced inside the can at high pressure during
manufacturing stages.
◦ That makes the propellant and the product mix together
◦ The propellant turns back to a gas when you push the nozzle and the pressure is released. It disappears into
the air leaving behind the product you want.
Gas Laws:
◦ Boyle’s Law is the absolute pressure exerted by a given mass of a gas is inversely proportional to the volume it
occupies if the temperature and amount of gas remain unchanged.
◦ When the volume increases, the pressure of the gas decreases in proportion.
◦ Boyle’s Law: In the aerosol can that have a lot of pressure in them, is what causes the liquid to come out when
pressure is applied to the can.
◦ Avogadro’s Law: equal volumes of all gases at the same temperature and pressure, have the same number of
molecules
◦ Under the same condition of temperature and pressure, equal volumes of all gases contain the same number of
molecules
◦ Avogadro’s Law: Until more pressure or heat is added to the aerosol can, the contents of the can stay the same
◦ Gay-Lussac’s Law:: At the same temperature and pressure, equal volumes of gas contains equal numbers of
molecules
◦ Gay-Lussac’s Law: Until more pressure or heat is added to the aerosol can, the contents of the can stay the same
Avagadro’s Law
Вам также может понравиться
- BuoyancyДокумент16 страницBuoyancyZdenkoОценок пока нет
- CAP The Journey of FlightДокумент646 страницCAP The Journey of Flightloicfreville50% (2)
- 16 - Air Conditionng Systems - Vaishnavi C. KambleДокумент13 страниц16 - Air Conditionng Systems - Vaishnavi C. KambleVaishnavi KambleОценок пока нет
- Uses and Applications of The Different Gas LawsДокумент8 страницUses and Applications of The Different Gas LawsWin7Addict100% (30)
- Building Hot Air BalloonДокумент11 страницBuilding Hot Air BalloonOkotie-Eboh ToritsejuОценок пока нет
- How Hot Air Balloons WorkДокумент21 страницаHow Hot Air Balloons WorkAtul GuptaОценок пока нет
- Gas Law: Boyle'S Law, Charles Law, Gay-Lussac'S Law. Combined Gas Law, and Ideal Gas LawДокумент91 страницаGas Law: Boyle'S Law, Charles Law, Gay-Lussac'S Law. Combined Gas Law, and Ideal Gas LawLee MedalloОценок пока нет
- Airworthiness DirectivesДокумент3 страницыAirworthiness DirectivesDuzzysОценок пока нет
- Charles LawДокумент12 страницCharles LawLerr Real RelleОценок пока нет
- EASA E Regulations Part 145Документ251 страницаEASA E Regulations Part 145chrysobergiОценок пока нет
- Japanese Balloon BombsДокумент94 страницыJapanese Balloon BombsCAP History Library100% (2)
- Jismo Science p3 2019Документ32 страницыJismo Science p3 2019Agnes de Young100% (6)
- Gas LawsДокумент20 страницGas Lawsapi-305343335Оценок пока нет
- Different Laws of Chemistry and Their Day ToДокумент40 страницDifferent Laws of Chemistry and Their Day ToSahejОценок пока нет
- Applications For Ideal Gas LawsДокумент6 страницApplications For Ideal Gas Lawsdreamxtreme16Оценок пока нет
- Air Conditioning Theory (Automotive)Документ21 страницаAir Conditioning Theory (Automotive)ingenierosunidosОценок пока нет
- ExamplesДокумент12 страницExamplesMoldir SerikОценок пока нет
- Boyles LawДокумент2 страницыBoyles LawjaОценок пока нет
- How A Hot Air Balloon Works and Its Major PartsДокумент1 страницаHow A Hot Air Balloon Works and Its Major PartsDarknessОценок пока нет
- Air Conditioning FinalДокумент5 страницAir Conditioning Finaladam hopeОценок пока нет
- Gay-Lussac's Law or Amonton's LawДокумент3 страницыGay-Lussac's Law or Amonton's LawRevilloОценок пока нет
- Compilation OF Different Gas LawsДокумент7 страницCompilation OF Different Gas LawsJean Angelove SantosОценок пока нет
- CharlessДокумент2 страницыCharlessRevilloОценок пока нет
- Charles SДокумент2 страницыCharles SRevilloОценок пока нет
- Chem Law LawДокумент2 страницыChem Law LawFaye Lianne MaderoОценок пока нет
- LawДокумент6 страницLawJaymart BobilesОценок пока нет
- Science 10Документ6 страницScience 10Andy ValdezОценок пока нет
- Untitled DocumentДокумент6 страницUntitled DocumentChukwuemeka OkoyeОценок пока нет
- Use The Law To Explain The Following ObservationsДокумент6 страницUse The Law To Explain The Following ObservationsCedric Adrian AblogОценок пока нет
- Gaseous State: Khoe Tjok TjinДокумент16 страницGaseous State: Khoe Tjok TjinLuna eukharisОценок пока нет
- Comfort Air ConditioningДокумент85 страницComfort Air Conditioningshrikant100% (6)
- Gay-Lussac's LawДокумент12 страницGay-Lussac's LawKyndra Maxynne CamachoОценок пока нет
- Science ScriptДокумент2 страницыScience ScriptTrixie San DiegoОценок пока нет
- Steam TrapДокумент5 страницSteam TrapMrinal Kanti RoyОценок пока нет
- How Does An External Combustion Engine Work?: Industrial RevolutionДокумент9 страницHow Does An External Combustion Engine Work?: Industrial RevolutionJomar TorrechillaОценок пока нет
- Emerald Green and Gold Elegant Applicant Cover LetterДокумент4 страницыEmerald Green and Gold Elegant Applicant Cover LetterSam Yvhanne Quinzon TanОценок пока нет
- Applications of Gas LawsДокумент3 страницыApplications of Gas LawsCharm GorospeОценок пока нет
- Kinetic Molecular TheoryДокумент4 страницыKinetic Molecular TheoryG-Ann N. BorjaОценок пока нет
- Practical ApplicationsДокумент3 страницыPractical ApplicationsELvin Jay CasiñoОценок пока нет
- Gay Lussac's Law in Real Life:: PressureДокумент2 страницыGay Lussac's Law in Real Life:: PressureMelanie Mijares EliasОценок пока нет
- The Working Processing of An AirДокумент1 страницаThe Working Processing of An Airanhtuan_bkОценок пока нет
- Inbound 2075202700053796033Документ1 страницаInbound 2075202700053796033geoffreypenaranda05Оценок пока нет
- LEARNING ACTIVITY SHEET 5 (Charles' Law)Документ2 страницыLEARNING ACTIVITY SHEET 5 (Charles' Law)cherrymaeregalario2001Оценок пока нет
- P V T P V T ConstantДокумент7 страницP V T P V T ConstantNoa BawieОценок пока нет
- Boyle's LawДокумент11 страницBoyle's LawDustin VazquezОценок пока нет
- Definition of Boyle's Law: What Is An Ideal Gas?Документ4 страницыDefinition of Boyle's Law: What Is An Ideal Gas?حسين كاظم ياسينОценок пока нет
- Noriel P. Padilla II 10 - Einstein Gas Laws Examples in Real LifeДокумент1 страницаNoriel P. Padilla II 10 - Einstein Gas Laws Examples in Real LifeAshley CastroОценок пока нет
- Chapter 4 - HeatДокумент23 страницыChapter 4 - HeatAnonymous IyNfIgG4Z100% (1)
- Chapter A 06 RefrigerationДокумент18 страницChapter A 06 RefrigerationСергей КороткийОценок пока нет
- Aux Mach 222 Midterm Week 4Документ14 страницAux Mach 222 Midterm Week 4Janoel LucañasОценок пока нет
- How Can I Make My NSTP Engagement Meaningful Sec39 DagcutanДокумент1 страницаHow Can I Make My NSTP Engagement Meaningful Sec39 DagcutanMaxine TaeyeonОценок пока нет
- Mechanical-Compression Refrigeration SystemsДокумент4 страницыMechanical-Compression Refrigeration Systemsaruna MoonОценок пока нет
- Soda BottleДокумент3 страницыSoda BottleBenedick CruzОценок пока нет
- Ideal Gas Law: Prepared By: Kristine Hazel W. Bidayan Special Science Teacher IДокумент29 страницIdeal Gas Law: Prepared By: Kristine Hazel W. Bidayan Special Science Teacher IThami CalaguiОценок пока нет
- Boyle's LawДокумент24 страницыBoyle's LawRalph Jasil Esparagoza EscanillasОценок пока нет
- How Does The Carburetor Works: A) TemperatureДокумент5 страницHow Does The Carburetor Works: A) TemperatureKhurram SherazОценок пока нет
- Boyles LawДокумент6 страницBoyles LawMara TillesОценок пока нет
- Final SciДокумент6 страницFinal SciDANAO, Izek Hyden P.Оценок пока нет
- Presentation 3Документ8 страницPresentation 3AKASHОценок пока нет
- Complete Vaporization Through Proper PreДокумент20 страницComplete Vaporization Through Proper PregalkaenОценок пока нет
- Role of Kinetic Theory of Gases in Vacuum Science and TechnologyДокумент14 страницRole of Kinetic Theory of Gases in Vacuum Science and Technologysehrish sharifОценок пока нет
- Vapor Compression Refrigeration CycleДокумент21 страницаVapor Compression Refrigeration CycleUSHA PAWARОценок пока нет
- How Does Air Condition WorksДокумент6 страницHow Does Air Condition Worksnguyen van ANОценок пока нет
- Gas LawsДокумент3 страницыGas LawsZynn Margarette DenostaОценок пока нет
- Chem301 Lab ManualДокумент42 страницыChem301 Lab ManualIreneVeladoОценок пока нет
- Olivia KaminskiДокумент2 страницыOlivia Kaminskiapi-305331033Оценок пока нет
- History BrochureДокумент2 страницыHistory Brochureapi-305331033Оценок пока нет
- Mole ProjectДокумент2 страницыMole Projectapi-305331033Оценок пока нет
- Frederick Douglass Essay ParagraphsДокумент4 страницыFrederick Douglass Essay Paragraphsapi-305331033Оценок пока нет
- Complete CapДокумент6 страницComplete Capapi-305331033Оценок пока нет
- The Wizard of Oz Populism EssayДокумент4 страницыThe Wizard of Oz Populism Essayapi-305331033Оценок пока нет
- Religion HeresyДокумент7 страницReligion Heresyapi-305331033Оценок пока нет
- GaulДокумент10 страницGaulapi-305331033Оценок пока нет
- History EssayДокумент4 страницыHistory Essayapi-305331033Оценок пока нет
- Math PaperДокумент1 страницаMath Paperapi-305331033Оценок пока нет
- Physics PendulumДокумент3 страницыPhysics Pendulumapi-305331033Оценок пока нет
- Hamlet Soundtrack ProjectДокумент21 страницаHamlet Soundtrack Projectapi-305331033Оценок пока нет
- Math ProjectДокумент3 страницыMath Projectapi-305331033Оценок пока нет
- Religion PrayerДокумент2 страницыReligion Prayerapi-305331033Оценок пока нет
- Gaul PowerpointДокумент7 страницGaul Powerpointapi-305331033Оценок пока нет
- Tkam PoemДокумент2 страницыTkam Poemapi-305331033Оценок пока нет
- GaulДокумент3 страницыGaulapi-305331033Оценок пока нет
- Ac RegДокумент33 страницыAc ReghitsansanthiОценок пока нет
- Ix HHW-4Документ42 страницыIx HHW-4Mayank GargОценок пока нет
- Test 5 For 7th GradersДокумент4 страницыTest 5 For 7th GradersDenis VrublevskijОценок пока нет
- Unit 1 Topic 2 Tutorial 1 Fluid - 2020Документ15 страницUnit 1 Topic 2 Tutorial 1 Fluid - 2020Bryan YeohОценок пока нет
- Balloons: Gas & Go - Questions: Directions: Answer The Questions After Reading The Article "Документ3 страницыBalloons: Gas & Go - Questions: Directions: Answer The Questions After Reading The Article "Kimberly TaboraОценок пока нет
- Science ScriptДокумент2 страницыScience ScriptTrixie San DiegoОценок пока нет
- Boyle's Law: 225mL 1.12atmДокумент4 страницыBoyle's Law: 225mL 1.12atmannmarieОценок пока нет
- Nghe-Viet 2Документ11 страницNghe-Viet 2nhat.taciturnОценок пока нет
- Aoe2104f06sampleexam1sol PDFДокумент4 страницыAoe2104f06sampleexam1sol PDFmgskumarОценок пока нет
- 3 Nelson - Up, Up, and Away! - AnswersДокумент3 страницы3 Nelson - Up, Up, and Away! - Answersdmccloy28Оценок пока нет
- Pre Flight ChecklistДокумент5 страницPre Flight ChecklistAbdur RahimОценок пока нет
- Tutorial 4 Fluid: Help University College 1Документ14 страницTutorial 4 Fluid: Help University College 1Yoke Hock SeowОценок пока нет
- Reading Unit 1.2Документ5 страницReading Unit 1.2Hoang ThaiОценок пока нет
- Hot Air BalloonsДокумент2 страницыHot Air BalloonsKheiren D. AriasОценок пока нет
- Lesson 10 Applications On Archimedes Principles Osunero, Kim John S. BSED 2DДокумент17 страницLesson 10 Applications On Archimedes Principles Osunero, Kim John S. BSED 2DKim John OsuneroОценок пока нет
- Modul KemДокумент44 страницыModul KemMohd ZainalОценок пока нет
- BMM1213 Statics Assignment Co1 (Chapter 2,3,4)Документ7 страницBMM1213 Statics Assignment Co1 (Chapter 2,3,4)Wan Nabila HusnaОценок пока нет
- Appendix GДокумент22 страницыAppendix Gqwrtp79100% (1)
- Density, Viscosity & Drag 2 QPДокумент13 страницDensity, Viscosity & Drag 2 QPSam JoeОценок пока нет
- DLP 2 Gas LawsДокумент2 страницыDLP 2 Gas LawsShielo Marie CabañeroОценок пока нет
- T TP 2660732 Lets Take A Trip Types of Transport Powerpoint Ver 4Документ13 страницT TP 2660732 Lets Take A Trip Types of Transport Powerpoint Ver 4Ксения ЯремчукОценок пока нет
- Density and Buoyancy: Main Menu Table of Contents BackДокумент20 страницDensity and Buoyancy: Main Menu Table of Contents BackNirmal JayanthОценок пока нет
- Annex 1 - Personnel Licensing: Transmittal NoteДокумент103 страницыAnnex 1 - Personnel Licensing: Transmittal Notekeerthi GopinathОценок пока нет